With reference to atomic number of one, explain why hydrogen can be placed in either group I and VII of the periodic table. K.N.E.C Marking Scheme
Hydrogen forms compounds by losing one electron like group I elements or by gaining one electron like group VII element /Hydrogen has one electron in outermost shell.
0 Comments
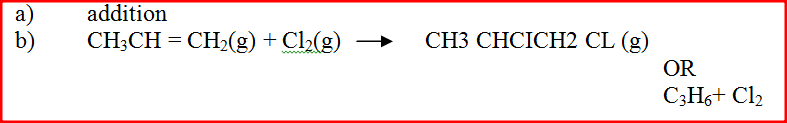
A Solution of chlorine in tetra chloromethane turns colourless when propene gas is bubble though it.
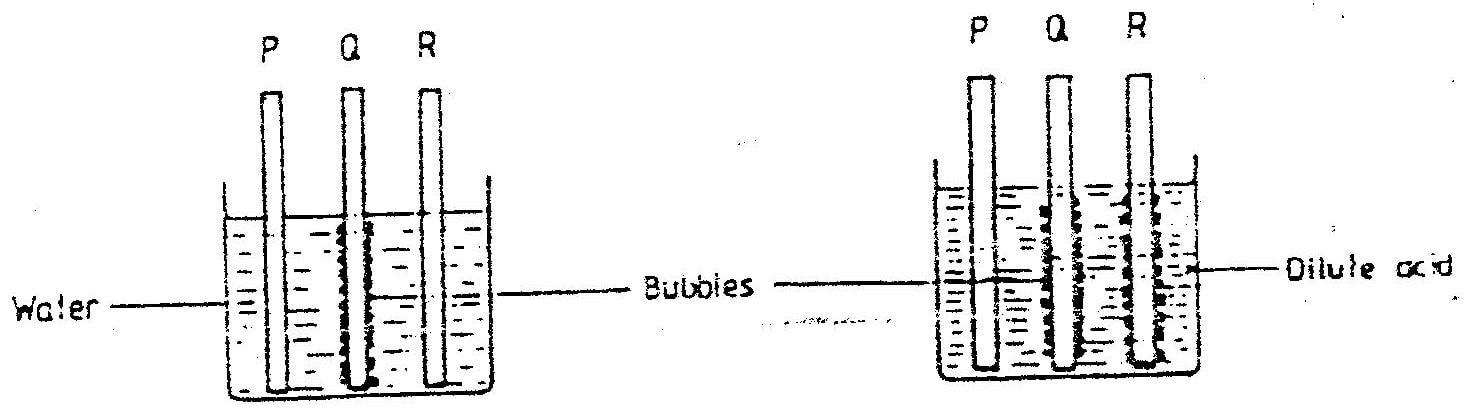
In an experiment, rods of metals P, Q and R were cleaned with sand paper and placed in a beaker containing water. Another set of rods was also cleaned and placed in a beaker containing dilute acid. After placing the rods in the two liquids bubbles of gas were seen around some of the rods as shown in the diagrams below.
K.N.E.C Marking Scheme
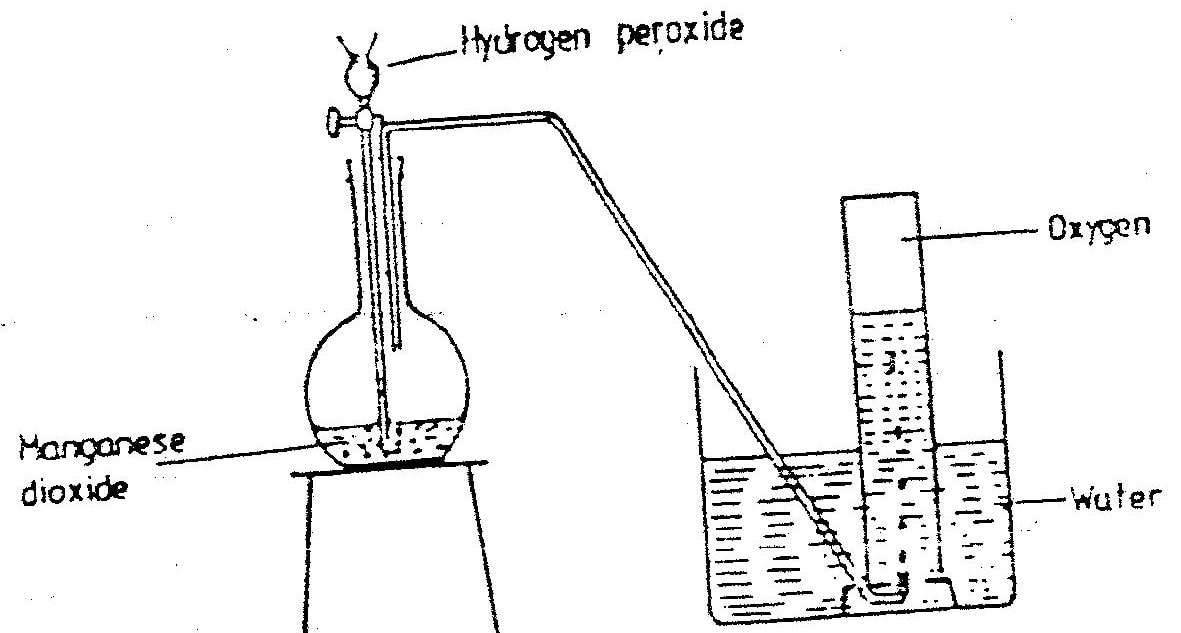
The diagram below represents a set–up that can be used to prepare and collect oxygen.
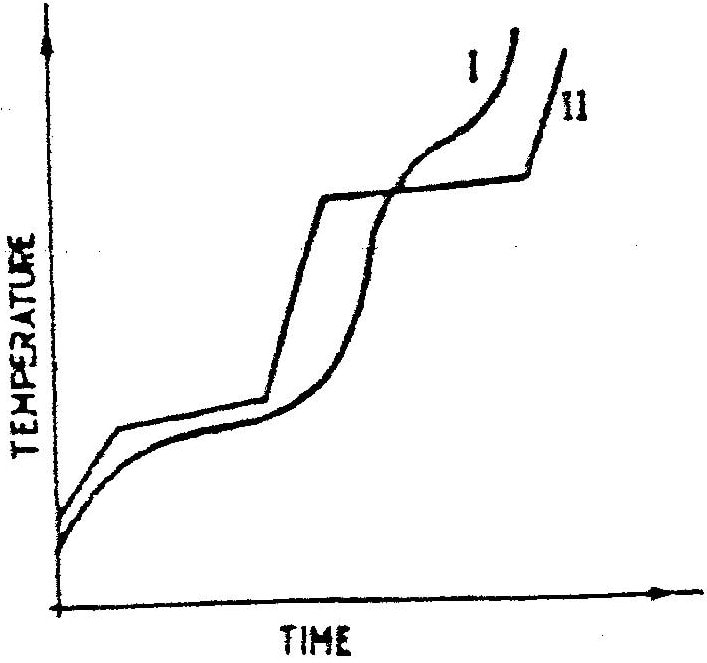
The curve below represents the variation of temperature with time when pure and impure samples of a solid were heated separately.Which curve sows the variation in temperature for the pure solid? Explain (2mks)
knec answers
II-Because pure substances have sharp MP and BP as shown by the flat regions of curve II. (accept systematic) (2 mks)
The table below gives some properties of gases D and E.
a) Name one natural fibre.
|
Chemistry Topics
All
Archives
December 2024
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
|
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |



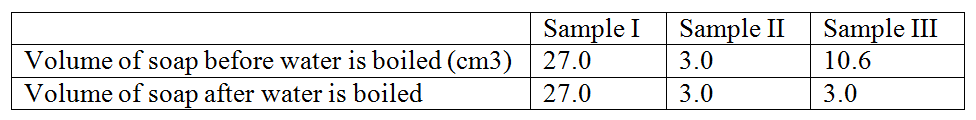


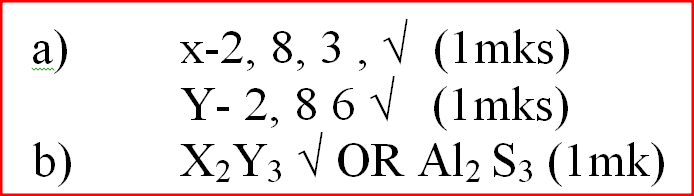

 RSS Feed
RSS Feed

