|
expected response
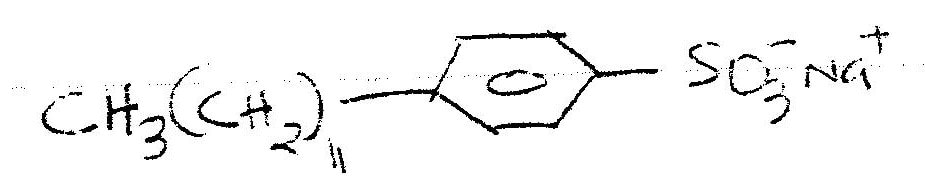
The ionic end lowers the surface tensions of water , facilitating mixing while the nonionic end (non-polar end) mixes with grease, dislodging it from the fabric.
0 Comments
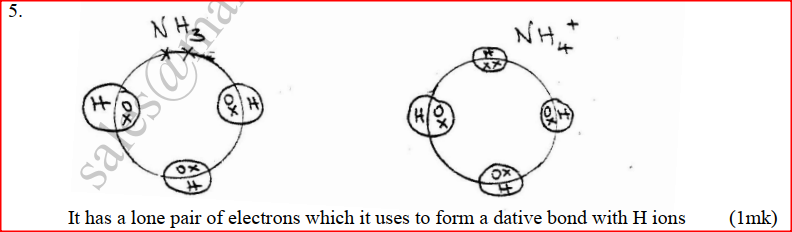
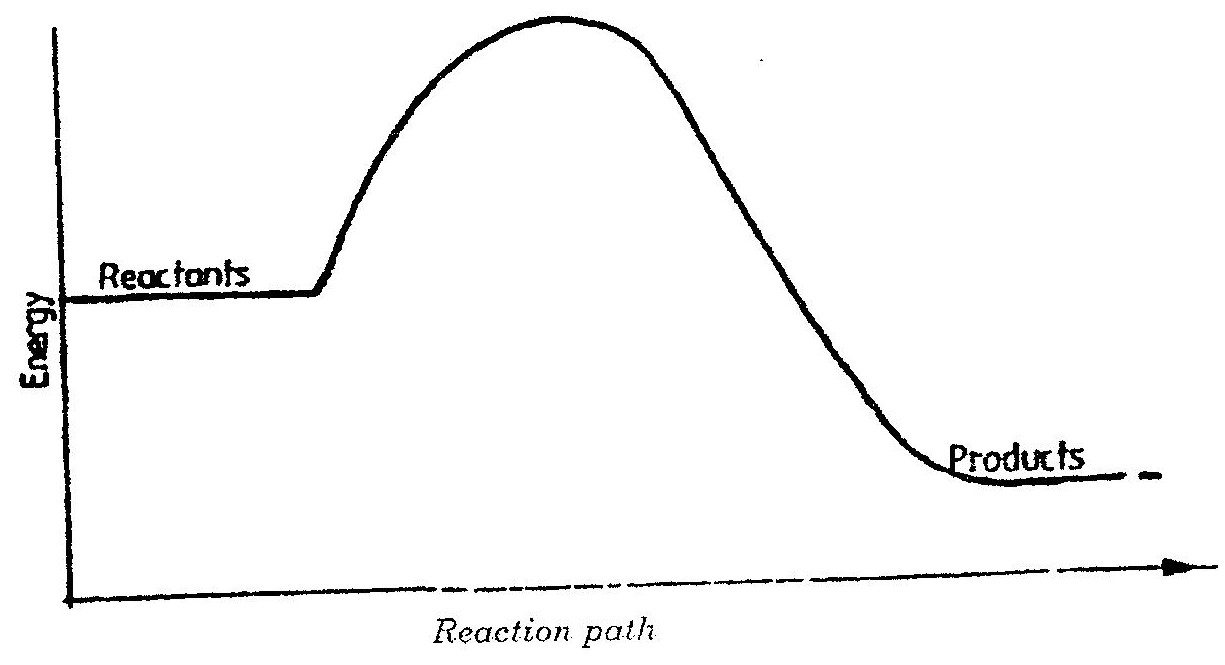
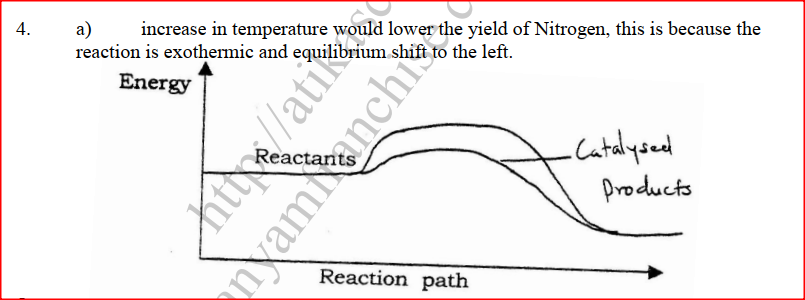
a) Using dots(.) and crosses (x) to represent electrons draw diagram to represent the bonding in: (i) NH3 (ii) NH4+ (1mk) b) State why an ammonia molecule (NH3) can combine with H+ to form NH4+ (Atomic numbers: N=7 and H=1) (1mk) Ammonia can be converted to nitrogen monoxide as shown in the equation below (a) Explain how an increase in temperature would affect the yield of nitrogen monoxide (2mks) (b) On the energy level diagram above sketch, the energy level diagram that would be obtained if the reaction is carried out in the presence of platinum catalyst. (1 mk)
expected response
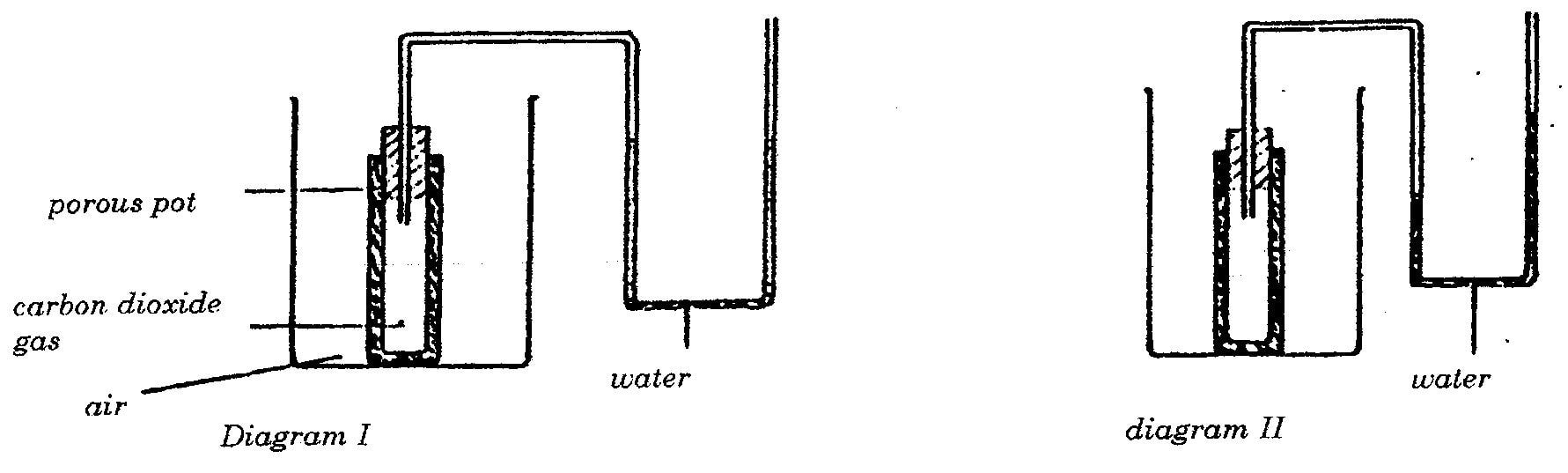
Air is less dense than carbon dioxide and so it enters the porous pot faster than carbon dioxide out of it. This sets up a higher pressure; in the pot and the level rises as shown:
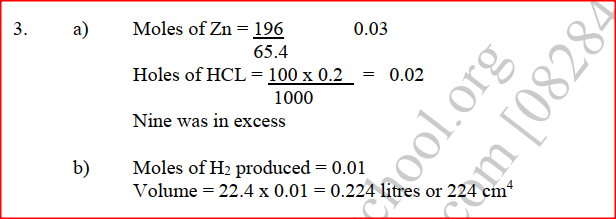
Zinc metal and hydrochloric acid reacts according to the following equation Zn(s) + 2HCI (aq)→ ZnCI2 (aq) + H2 (g) 1.96 g of zinc were reacted with 100cm3of 0.2M hydrochloric acid (a) Determine the reagent that was in excess (b) Calculate the total volume of hydrogen gas was liberated S.T.P (Zn= 65.4 Molar gas volume = 22.4 litres at S.T.P) A fixed mass of a gas has a volume of 250cm3 at a temperature of 270C and 750mm Hg pressure. Calculate the volume the gas would occupy at 420C (2mks)
answer
(a) Solid dissolves
(b) addition of hydrochloric acid favour backward reaction
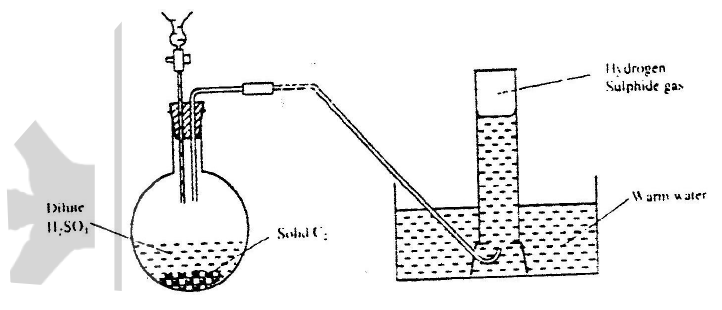
Describe how a solid sample of Zin (II) carbonate can be prepared starting with zinc oxide
The structures below represents a portion of a polymer
(a) Give the name of the polymer (b) Give one industrial use of the polymer
answer
With reference to iodine, distinguish between covalent bonds and Van Der Waals forces
answers
When carbon dioxide gas was passed through aqueous calcium hydroxide a white suspension was formed
(a) Write an equation for the reaction that took place (b) State and explain the changes that would occur when carbon dioxide gas is bubbled through the white suspension
Iron is extracted from its ore by the blast furnace process
(a) Name one ore from which iron is extracted (b) One of the impurities in iron is removed in the form of calcium silicate. Write an equation for the reaction in which calcium silicate is produced
Concentrated nitric (V) acid was added to iron (II) sulphate acidified with sulphuric acid and the mixture heated. The solution turned from pale green to yellow with evolution of brown gas. Explain these observations.
answer
In an experiment, sulphur dioxide gas was bubbled into water followed by chlorine gas. The resulting clear solution gave a white precipitate when mixed with a acidified barium chloride solution. Explain these observations
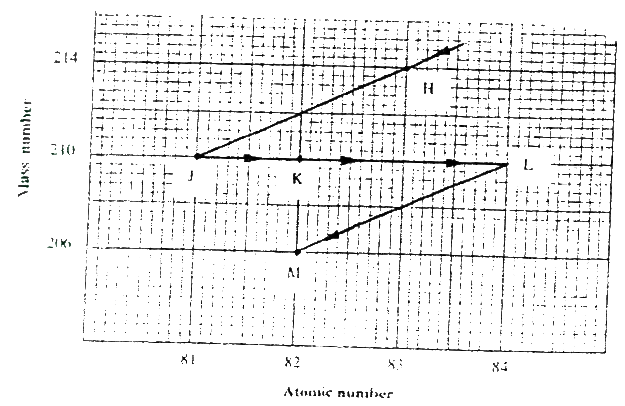
The graph below represents a radioactive decay series for isotope H. Study it and answer the questions that follow
(a) Name the type of radiation emitted when isotope H changes to isotope J.
(b) Write an equation for the nuclear reaction that occur when isotope J changes to isotope K
(c) Identify a pair of isotope of an element in the decay series
Use the reactions given below to answer the questions that follow. The letters do not represent the actual symbols of the elements
(a) What name is given to the type of reaction given above?
(b) Arrange the elements D, E, F and G in the order of their reactivity starting with the most reactive (c) Complete the equation below
answers
State and explain how the rate of reaction between zinc granules and steam can be increased
answer
When potassium nitrate is heated, it produces potassium nitrate and gas C
(a) Identify gas C (b) Name the type of reaction undergone by the potassium nitrate
answer
(a) Write the electronic configuration of calcium ( atomic number 20 ) and beryllium (atomic number 4)
(b) Why is calcium more reactive than beryllium
answers
The following two tests were carried out on chlorine water contained in two test tubes
(a) A piece of blue flower was dropped into the first – tube. Explain why the flower was bleached (b) The second test- tube was corked and exposed to sunlight after a few days, it was found to contain a gas that rekindled a glowing splint. Write an equation for the reaction which produced the gas
Give one example of elements A and B
A B
ANSWER
Oxygen and sulphur belong to group (VI) of the periodic table. Explain why there is a big difference their (melting points of oxygen is –2160C while that of sulphur is 440C.
ANSWER
Name One property of neon that makes it possible to be used in electric lamps
ANSWERS
|
Chemistry Topics
All
Archives
December 2024
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
|
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |

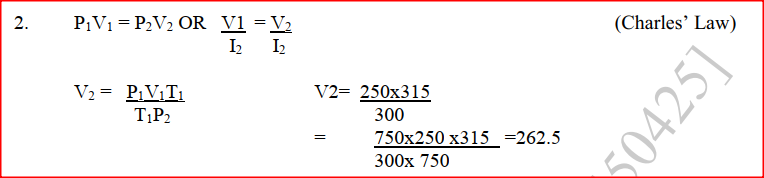

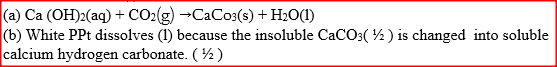
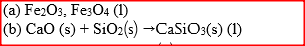



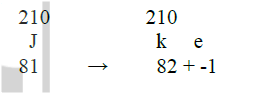
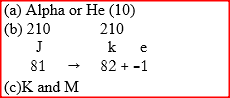
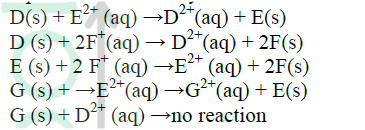
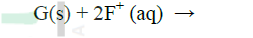


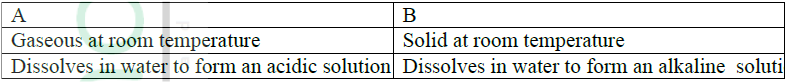

 RSS Feed
RSS Feed

