|
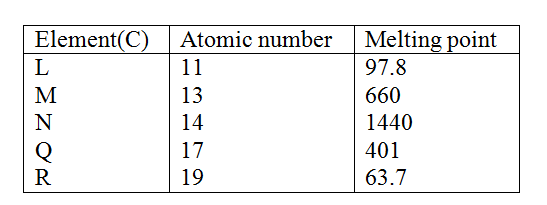
Study the information in the table below and answer the questions that follow. The letters do not represent the symbols of the elements.
a) Write the electrons arrangement for the atom formed by elements and M and Q
b) Select an element which is
d) Element R lodes its outermost electron more readily than I. Explain e) Using dots(.) and crosses (x) to represent outermost electrons show bonding in the compound formed elements N and Q. f) Explain why the melting point elements M is higher than that of element . g) Describe how a solid mixture of sulphate of R and lead sulphate can be separated into solid samples.
0 Comments
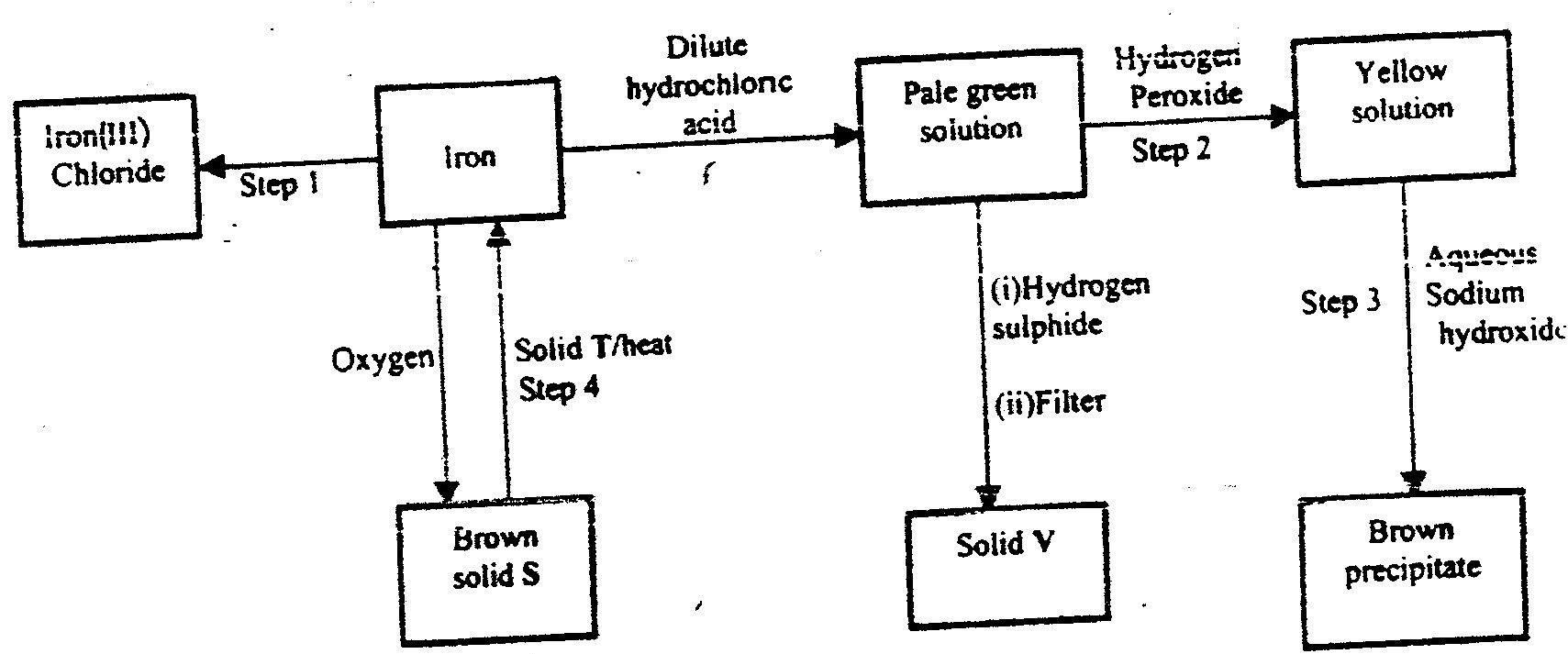
a) The flow chart below shows a sequence of reactions starting with.
Study and answer the questions that follow.
Cu = 63.5, Fe = 56.0
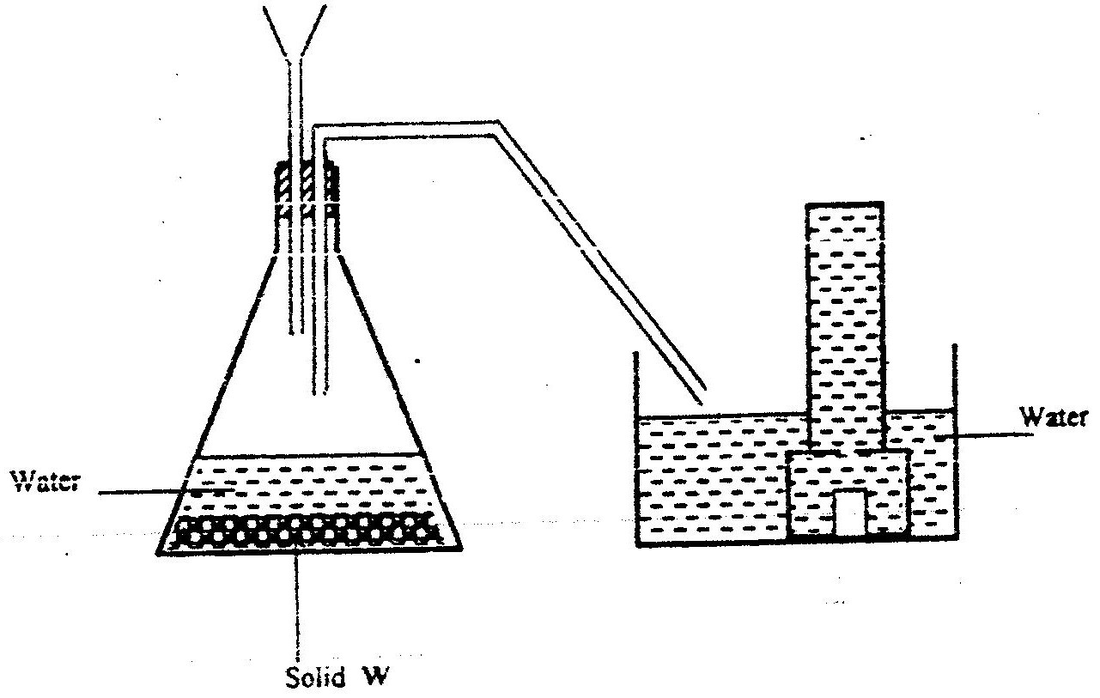
a) The diagram below shows a set –up used by as a student in an attempt to prepare and collect oxygen gas
c) The reaction between sulphur dioxide and oxygen to form trioxide in the contact process in exothermic. Factory manufacturing sulphuric acid by contact process produces 350kg of sulphur trioxide per day (conditions) for the reaction catalyst. 2 atmospheres pressure and temperatures between. (400 – 5000C) i) What is meant by an exothermic reaction? ii) How would the yield per day of sulphur trioxide be affected Temperatures lower than 40000C are used? Explain.
a) When an organic compound Y is reacted with aqueous sodium carbonate, it produces carbon dioxide reacts with propanol to form a sweet smelling compound Z whose formula is.
c) The scheme below was used to prepare a cleaning agent. Study and answer the questions that follow.
i) What name is given to the type of cleaning agent prepared by the method shown in the scheme?
ii) Name one chemical substance added in step II iii) What is the purpose of adding the elements substance names in C(ii) above. iv) Explain how an aqueous solution of the cleansing removes oil from utensils during washing.
10cm3 of concentrated sulphuric (VI) acid was diluted to 100cm3. 10cm3 of the Resulting solution was neutralised by 36cm3 of 0.1M sodium hydroxide solution. Determine the mass of sulphuric (VI) acid that was in the concentrated acid (S = 32.0; H = 1.0; O = 16.0).
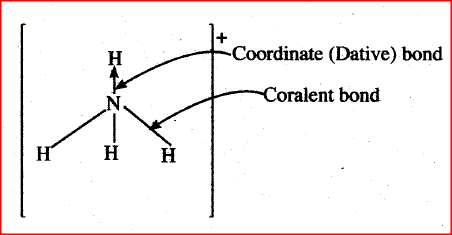
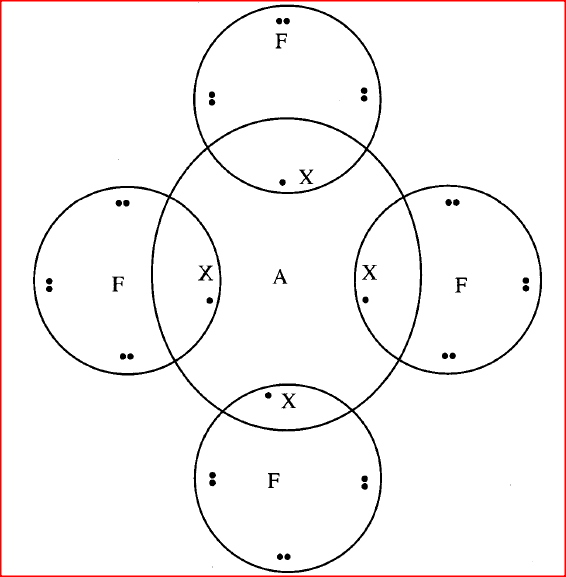
Ammonium ion has the following structure:
Label on the structure:
(a) covalent bond;
(b) coordinate (dative) bond.
Study the information in the table below and answer the questions that follow: A mixture containing 35g of CuS04 and 78g of Pb(N03)2 in l00g of water at 60°C was cooled to 40°C.
(a) Which salt crystallised out? Give a reason
(b) Calculate the mass of the salt that crystallised out.
A sample of water in a beaker was found to boil at 101.5°C at 1 atmospheric pressure.
Assuming that the thermometer was not faulty, explain this observation.
ANSWERS
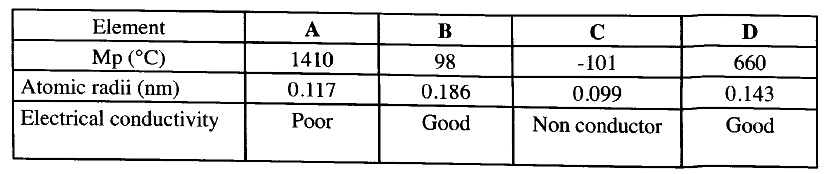
The table below shows properties of some elements A, B, C and D which belong to the same period of the periodic table. The letters are not the actual symbols of the elements.
(a) Arrange the elements in the order they would appear in the period. Give a reason.
(b) Select the metallic element which is the better conductor of electricity. Give a reason.
ANSWERS
(a) BDACAcross the period the atomic radius decreases.
(b) D Across the period the conductivity increase due to increase in delocalised electrons.
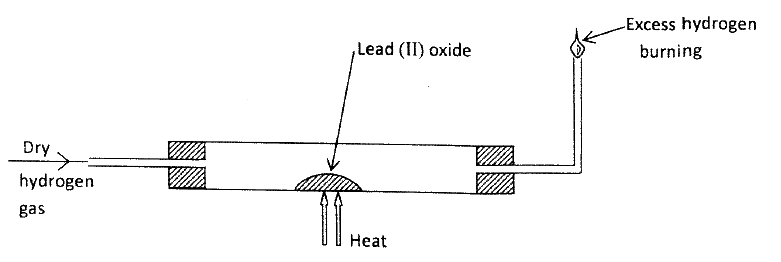
In an experiment, dry hydrogen gas was passed over heated Lead (II) Oxide as shown in the diagram below.
State and explain the observations made in the combustion tube.
ANSWERS
Iron (III) oxide was found to be contaminated with copper (II) sulphate. Describe how a pure sample of iron (III) oxide can be obtained.
Charcoal is a fuel that is commonly used for cooking. When it burns it forms two oxides.
(a) Name the two oxides. (b) State one use of any of the two oxides.
ANSWERS
(a) Carbon (IV) Oxide and Carbon (II) Oxide
(b) CO2- Fire extinguishers Fizzy drinks Food preservative . Solvay process . CO -Manufacture of fuel (water gas) Reduction in the extraction of metals Manufacture of methanol
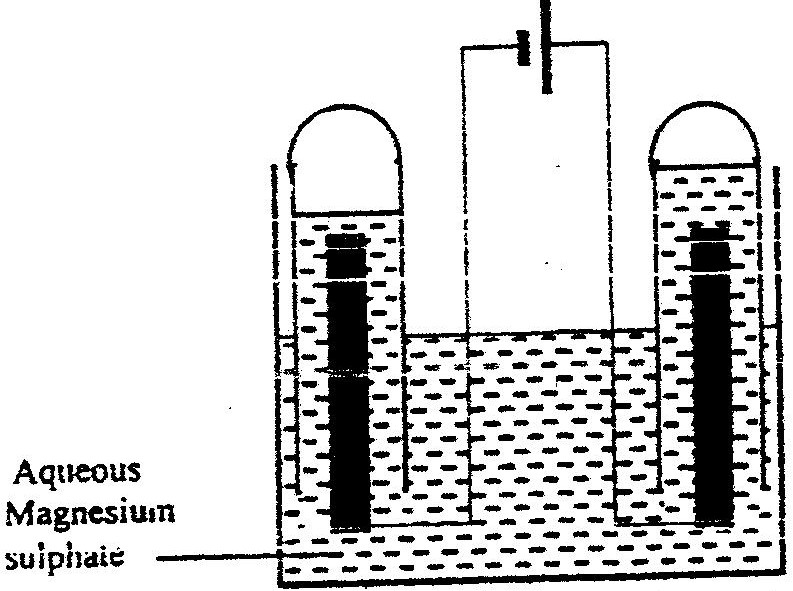
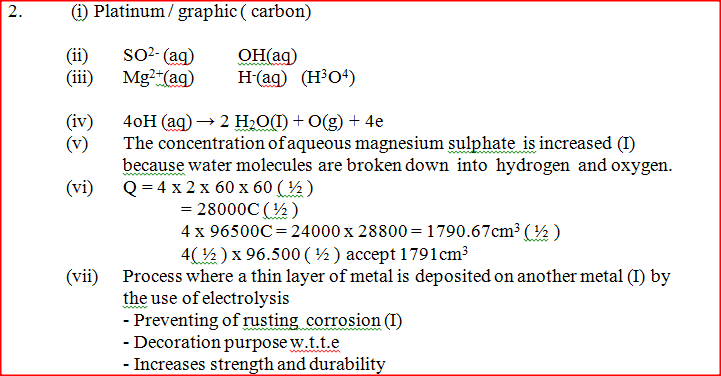
The set – up below was used during the electrolysis of aqueous magnesium sulphate using inert electrodes
i) Name a suitable pair of electrodes for this experiment ii) Identify the ions and cations in the solution iii) On the diagram label the cathode iv) Write ionic equations for the reactions that took place at the anode. v) Explain the change that occurred to the concentration of magnesium sulphate solution during the experience. vi) During the electolysis a current of 2 amperes was passed through the solution for 4 hours. Calculate the volume of the gas produced at the anode.(1 faraday 96500 coulombs and volume of a gas at room temperature is 24000cm3) vii) One of the uses of electrolysis is electroplating What is meant by electroplating? Give tow reasons why electroplating is necessary.
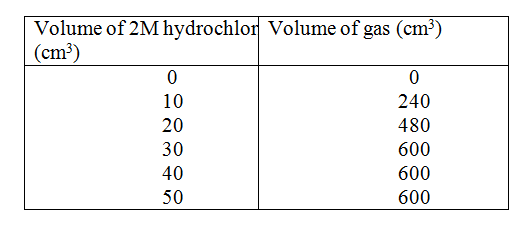
The table below gives the volume of the gas provided when different volumes of 2M hydrochloric were reacted with 0.6g of magnesium powder at room temperature
(a) Write an equation fro the reaction between magnesium and hydrochloric acid
(b) On the grid provided plot a graph of the volume of gas produced (vertical axis), against the volume of acid added (Note the reaction comes to completion, the volume of the gas produced directly proportional to completion, the acid added). From the graph determine c)
e) Given that one mole of the gas occupied 24000cm3 at room temperature. The table below shows the PH values of solutions I, II, III and IV.
(a) Which solution is likely to be that of calcium hydroxide?
(b) Select the solution in which a sample of aluminium oxide is likely to dissolve. Give reason for your answer
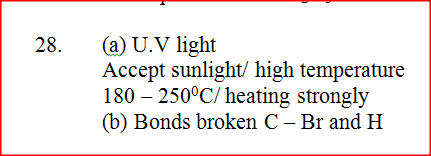
Bromine reacts with ethane as shown below
(a) What condition is necessary for this reaction to occur?
(b) Identify the bonds which are broken and those that are formed
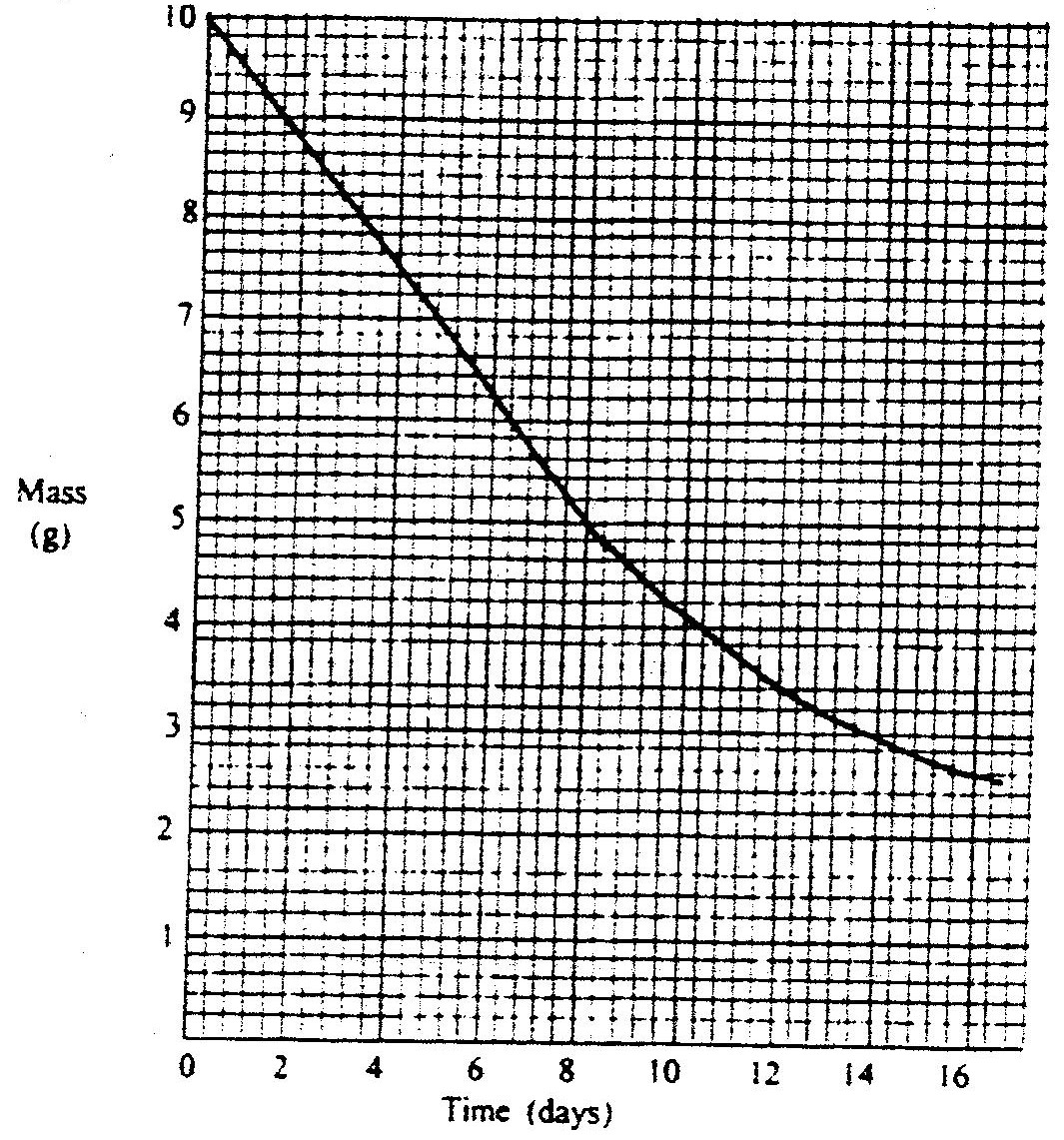
The graph below shows the mass of a radioactive isotope plotted against time
(a) Using the graph, determine the half – life of the isotope
(b) Calculate the mass of the isotope present after 32 days
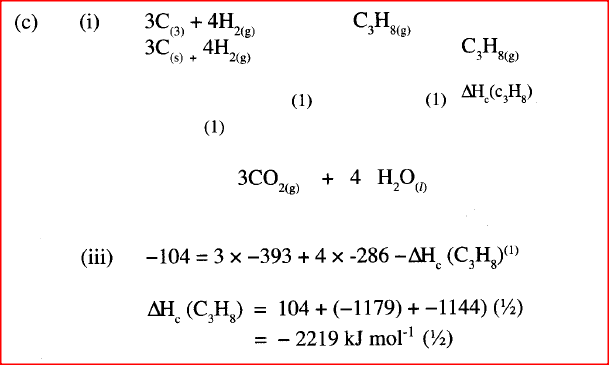
(a) What is meant by molar heat of combustion?
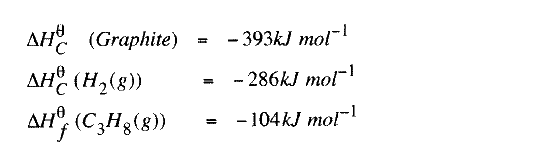
(b) State the Hess's Law. c) Use the following standard enthalpies of combustion of graphite, hydrogen and enthalpy of formation of propane.
(i) Write the equation for the formation of propane.
(ii) Draw an energy cycle diagram that links the heat of formation of propane with its heat of combustion and the heats of combustion of graphite and hydrogen. (iii) Calculate the standard heat of combustion of propane. Other than the enthalpy of combustion, state one factor which should be considered when choosing a fuel. (e) The molar enthalpies of neutralization for dilute hydrochloric acid and dilute nitric (V) acid are -57.2kJ/mol while that of ethanoic acid is -55.2kJ/mol. Explain this. observation.
ANSWERS
(a) The amount of heat liberated when one mole of a substance is burnt in excess Oxygen.
(b) The heat evolved are absorbed in a chemical change is the same whether the change occurs in one step or through many steps.
(d) cost
effect on environment availability storage (e) Ethanoic acid is a weak acid therefore heat is used to ionize it before neutralization occurs . It value is therefore lower than that of hydrochloric acid which is fully ionized
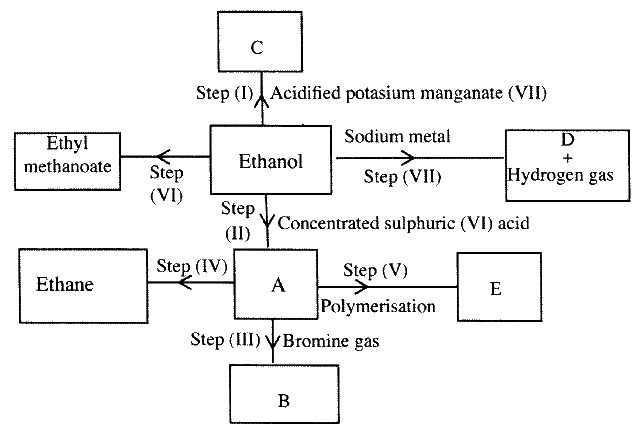
(a) Study the flowchart below and answer the questions that follows;
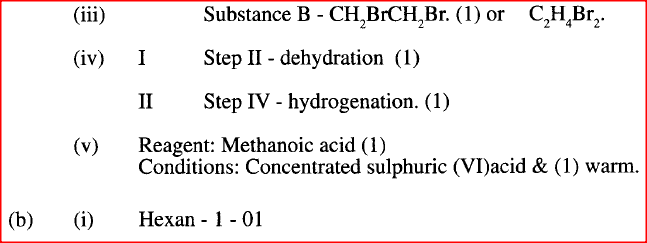
(i) I What observation will be made in Step I
II Describe a chemical test that can be carried out to show the identity of compound C (ii) Give the names of the following I E II substance D (iii)Give the formula of substance B . (iv) Name the type of reaction that occurs in: I Step (II) II Step (IV) (v) Give the reagent and conditions necessary for Step (VI). Reagent: Conditions (b) (i) Name the following structure.
(ii) Draw the structure of an isomer of pentene.
ANSWERS
(a) (i) I The potassium permanganate is decolorized or changes from purple to colourless.
II C is a ethanoic acid (carboxylic acid) Add sodium carbonate, you will see effervescence, test gas evolved with lime water, it will form a white precipitate. (ii) I Polyethene II Substance D - sodium ethoxide
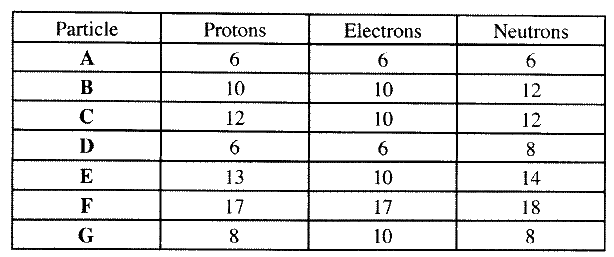
(a) Other than their location in the atom, name two other differences between an electron and a proton.
(b) the table below gives the number of electrons ,protons and neutrons in particles A,B, C , D, E, F and G
(i) Which particle is likely to be a halogen?
(ii) what is the mass number of E (iii) write the formula of the compound formed when E combines with G iv) Name the type of bond formed in (iii) above. (v) How does the radii of C and E compare ? Give reason. (vi) Draw a dot (.) and cross(x) diagram for the compound formed between (vii) Why would particle B not react with particle D?
ANSWERS
(a)- electron has I mass while proton has mass of one mass unit. 1840
- proton is positively charged while electron is negatively charged.
(iv)Ionic bond or electrostatic
(v)E has a smaller atomic radius than C E has more protons than C : nuclear attraction stronger.
(vii)Particle B is inert with a stable electronic configuration will not react.
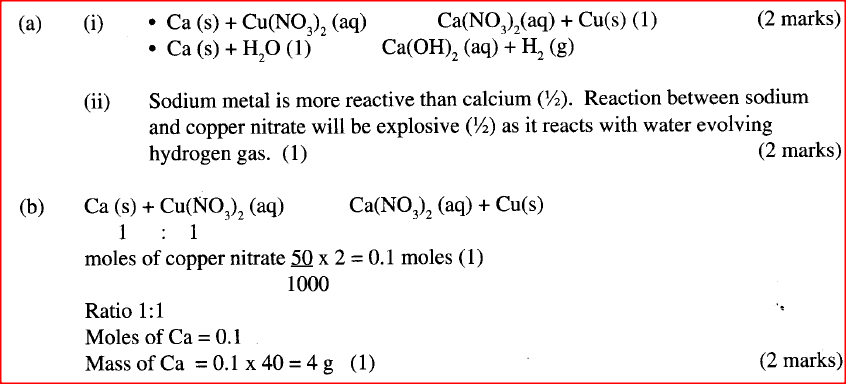
(a) When excess calcium metal was added to 50 cm3 of 2 M aqueous copper (II) nitrate in a beaker, a brown solid and bubbles of gas were observed.
(i) "Write two equations for the reactions which occurred in the beaker. (ii) Explain why it is not advisable to use sodium metal for this reaction.
(b) Calculate the mass of calcium metal which reacted with copper (II) nitrate solution. (Relative atomic mass of Ca = 40)
(c) The resulting mixture in (a) above was filtered and sodium hydroxide added to the filtrate dropwise until in excess. What observations were made? (d) (i) Starting with calcium oxide, describe how a solid sample of calcium carbonate can be prepared. (ii) Name one use of calcium carbonate
ANSWERS
(c)A white precipitate is formed which is insoluble in excess.
(d) (I) Add dilute nitric (V) acid to calcium oxide to form the soluble salt calcium nitrate. Add sodium carbonate (another soluble salt) to form insoluble. Calcium Carbonate and sodium nitrate . Filter out the calcium carbonate, wash it with distilled water to remove traces of sodium nitrate and dry between filter papers (ii) Manufacture of cement Manufacture of sodium carbonate.
(a) Ethanol can be manufactured from ethene and steam as shown in the equation below:
Temperature and pressure will affect the position of equilibrium of the above reaction. Name the other factor that will affect the position of equilibrium of the above reaction.
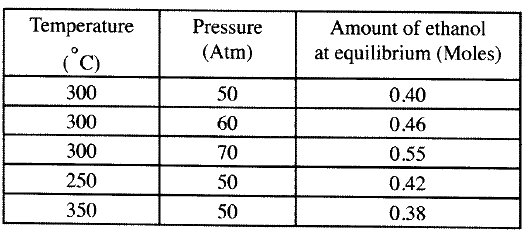
(b) The data in the table below was recorded when one mole of ethene was reacted with excess steam. The amount of ethanol in the equilibrium mixture was recorded under different conditions of temperature and pressure. Use the data to answer the questions that follow.
(i)State whether the reaction between ethene and steam is exothermic or endothermic.
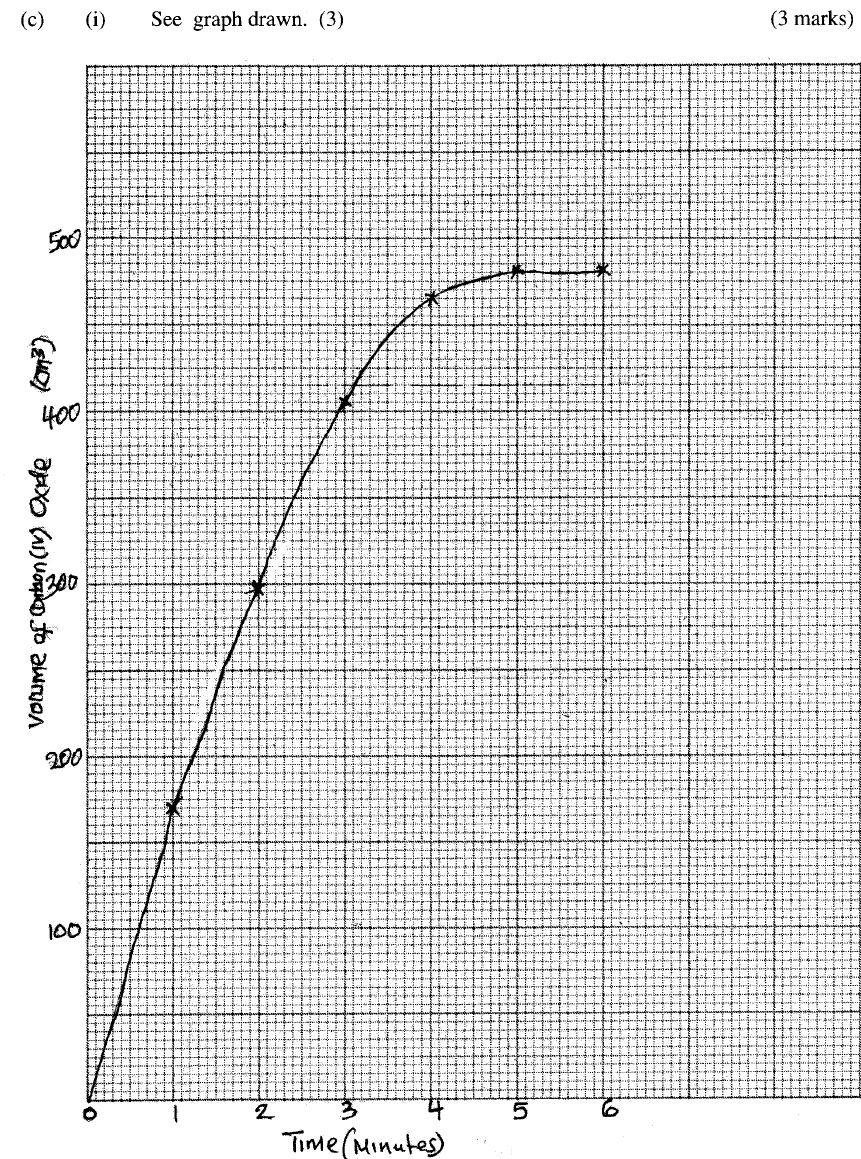
Explain your answer. (ii) State and explain one advantage and one disadvantage of using extremely high pressure in this reaction. I Advantage II disadvantage (c) In an experiment to determine the rate of reaction between calcium carbonate and dilute hydrochloric acid, 2g of calcium carbonate were reacted with excess 2 M hydrochloric acid, The volume of carbon (IV) oxide evolved was recorded at regular intervals of one minute for six minutes. The results are shown in the table below.
(i) plot a graph of time in minutes on the horizontal axis against volume of carbon (IV) oxide on the vertical axis.
(ii) determine the rate of reaction at 4 minutes
ANSWERS
(a) Increase or change in amount of reagent either reactants or products.
(Concentration). (b) (i) Exothermic increase in temperature from 250 - 350 at constant pressure the amount of ethanol formed at equilibrium decreases. (ii) I Advantage - it would increase the yield of ethanol ; since increase in pressure will favour side with less moles i.e. the products. II Disadvantage - it would mean investment in equipment to withstand the high pressure and would be expensive.
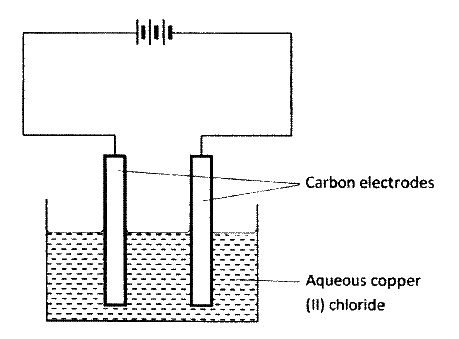
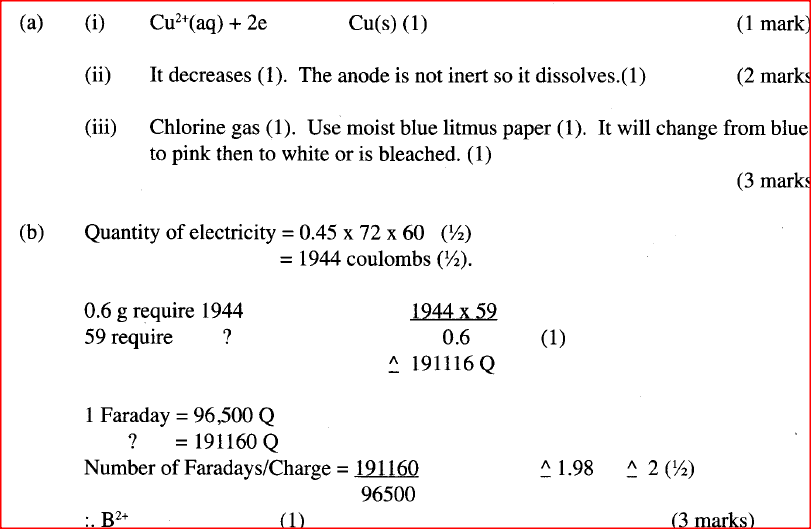
The set-up below was used by a student to investigate the products formed when aqueous copper (II) chloride was electrolyzed using carbon electrodes.
(a) (i) Write the equation for the reaction that takes place at the cathode.
(ii) Name and describe a chemical test for the product initially formed at the anode when a highly concentrated solution of copper (II) chloride is electrolysed. (iii) How would the mass of the anode change if the carbon anode was replaced With copper metal? Explain. (b) 0.6 g of metal B were deposited when a current of 0.45A was passed through an electrolyte for 72 minutes. Determine the charge on the ion of metal B. (Relative atomic mass of B = 59, 1 Faraday = 96 500 coulombs) (c) The electrode potentials for cadmium and zinc are given below:
Explain why it is not advisable to store a solution of cadmium nitrate in a container made of zinc
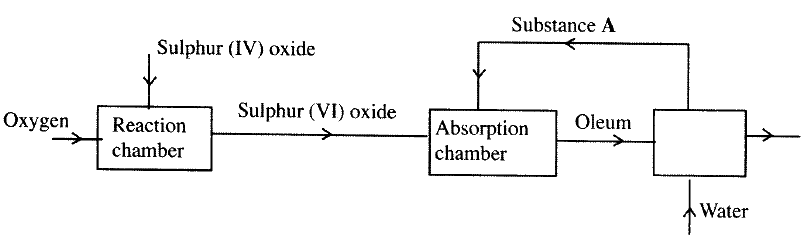
The flow chart below shows some of the processes involved in large scale production of sulphuric (VI) acid. Use it to answer the questions that follow.
a) Describe how oxygen is obtained from air on a large scale
(b) (i) Name substance A. (ii)Write an equation for the process that takes place in the absorption chamber. (c) Vanadium (V) oxide is a commonly used catalyst in the contact process. (i) Name another catalyst which can be used for this process. (ii) Give two reasons why vanadium (V) oxide is the commonly used catalyst. (d) State and explain the observations made when concentrated sulphuric (VI) acid is added to crystals of copper (II) sulphate in a bearer. (e) The reaction of concentrated sulphuric (VI) acid with sodium chloride produces hydrogen chloride gas. State the property of concentrated sulphuric (VI) acid , illustrated in this reaction. (f) Name four uses of sulphuric (VI) acid
What name is given to elements which appear in group (II) of the periodic table?
ANSWER
|
Chemistry Topics
All
Archives
December 2024
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
|
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |
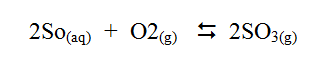
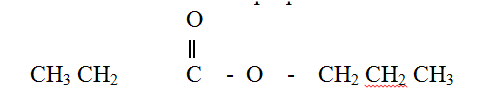


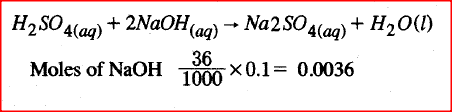
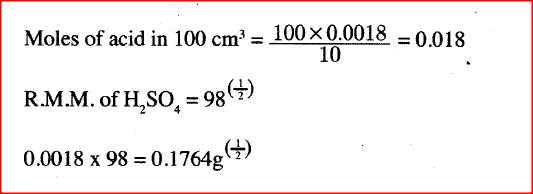

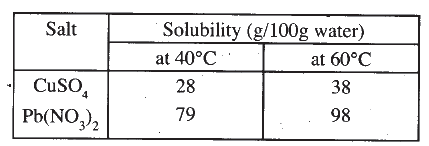

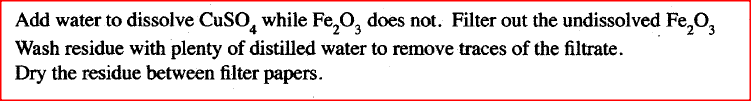

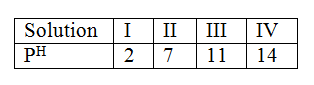

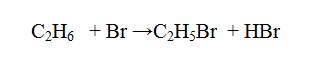
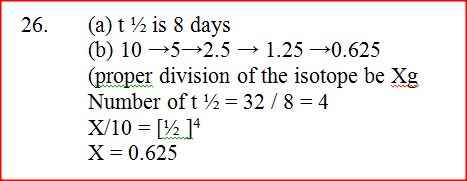
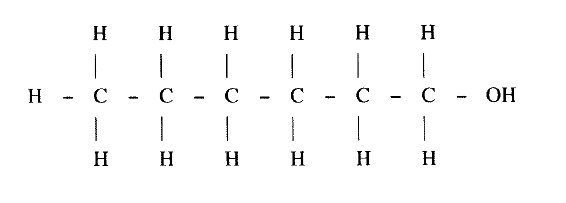
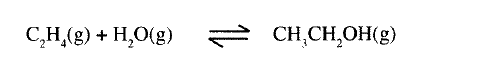

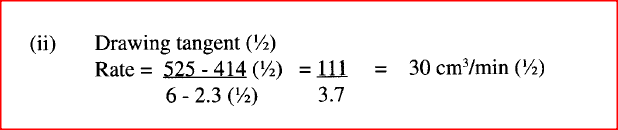
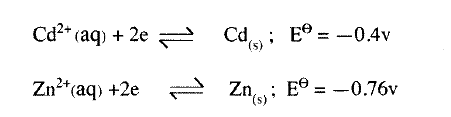

 RSS Feed
RSS Feed

