|
(a) In Kenya, sodium carbonate is extracted from trona at Lake Magacli.
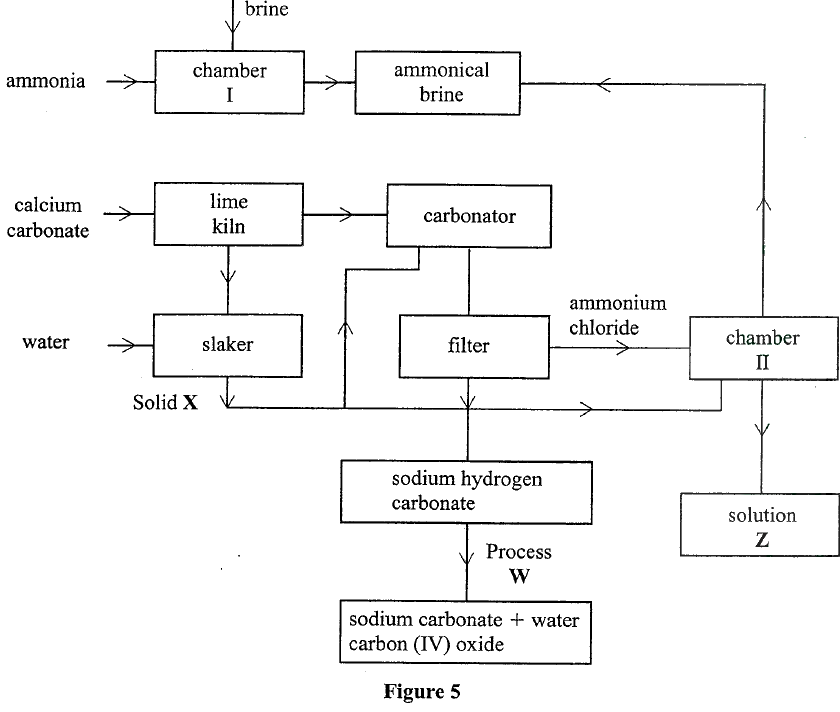
(i) Give the formula of trona. (ii) Name the process of extracting sodium carbonate from trona. (b) The flow chart in Figure 5 summarises the steps involved in the production of sodium carbonate. Use it to answer the questions that follow.
(i) Name the process illustrated in Figure 5.
(ii) Identify the starting raw materials required in the production of sodium carbonate. (iii) Write equations for the two reactions that occur in the carbonator. (iv) Name two substances that are recycled. (v) Identify: Solid X; Process W. (vi) Write an equation for the reaction that produces solution Z. (vii) Apart from softening hard water, state two other uses of sodium carbonate.
0 Comments
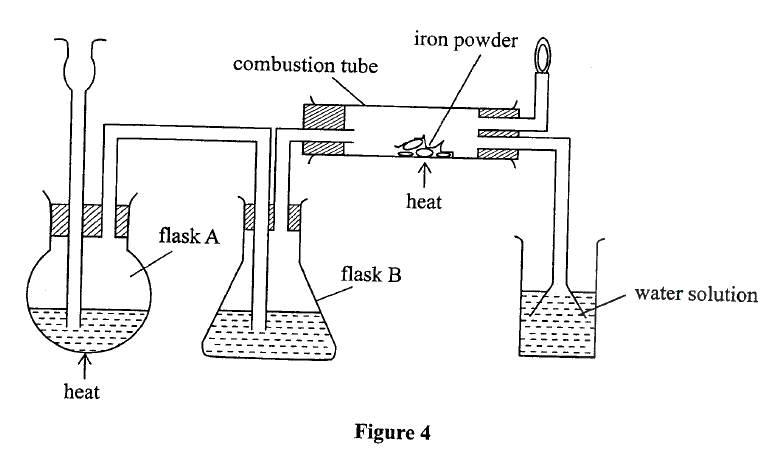
(a) The diagram in Figure 4 was used to prepare hydrogen chloride gas which was passed over heated iron powder.
(i) Give a pair of reagents that will produce hydrogen chloride gas in flask A.
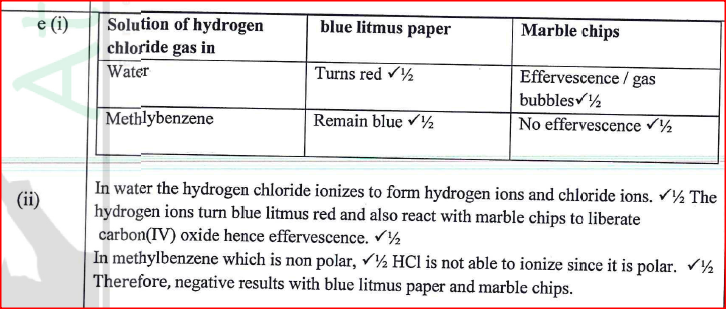
(ii) Name the substance in flask B. (iii) State the observation made in the combustion tube. (iv) Write an equation for the reaction in the combustion tube. (v) Describe a chemical test for hydrogen chloride gas. (b) (1) Identify the gas that burns at the jet. (ii) Explain why the gas in (b) (I) is burned. (c) Give reasons why excess hydrogen chloride gas is dissolved using the funnel arrangement. (d) State what will be observed when the reaction in the combustion tube is complete. (e) Another experiment was carried out where hydrogen chloride gas was bubbled through methylbenzene and water in separate beakers. The resulting solutions were tested with blue litmus papers and marble chips. (i) Write the observations made in the following table.
(ii) Explain the observations in (e) (i).
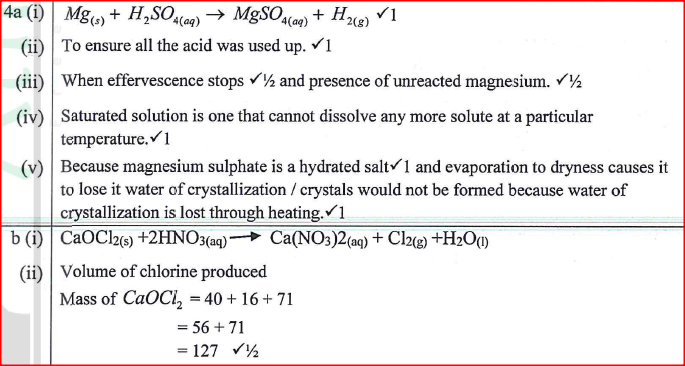
An experiment was carried out to prepare crystals of magnesium sulphate. Excess magnesium powder was added to 100 cm3 of dilute sulphuric (VI) acid in a beaker and warmed until no further reaction took place. The mixture was filtered and the filtrate evaporated to saturation, then left to cool for crystals to form.
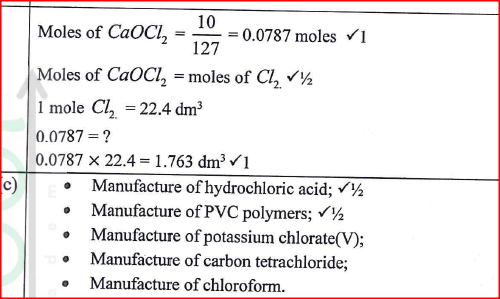
(a) (i) Write an equation for the reaction. (ii) Explain why excess magnesium powder was used. (iii) State how completion of the reaction was determined. (iv) What is meant by a saturated solution? (v) Explain why the filtrate was not evaporated to dryness. (b) When bleaching powder, CaOCl2, is treated with dilute nitric(V) acid, chlorine gas is released. This reaction can be used to determine the chlorine content of various samples of bleaching powders and liquids. (i) Write an equation for the reaction of nitric(V) acid with bleaching powder. (ii) Calculate the volume of chlorine produced when 10g of CaOCl2 is treated with excess nitric (v) acid. (Ca = 40.0; 0 = 16.0; Cl = 35.5; 1 mole of gas occupies 22.4dm3 at s.t.p) (c) Apart from use of chlorine gas in bleaches and water treatment, state two other uses of chlorine gas.
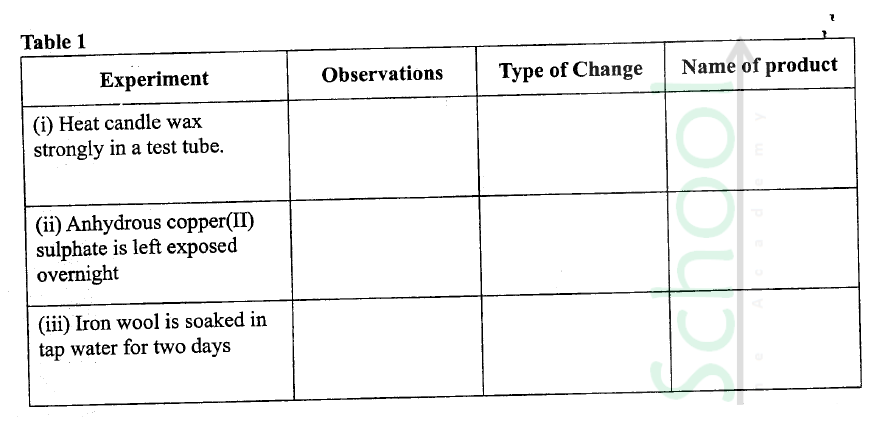
(a) Complete Table I by indicating the observations, type of permanent or temporary change and name of new compound formed.
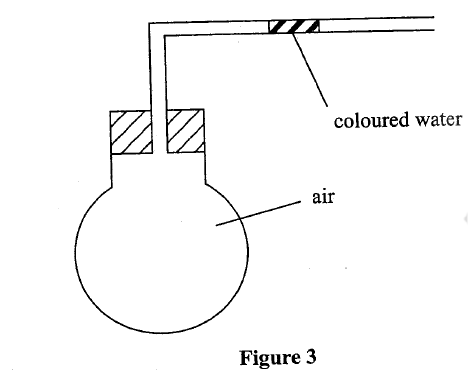
(b) Use the set-up in Figure 3 to answer the questions that follow. The flask was covered with a cloth that had been soaked in ice-cold water.
(i)State the observation made on the coloured water. Explain.
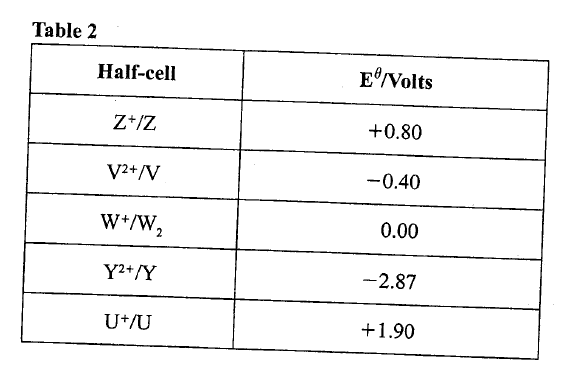
(ii) Name the gas law illustrated in Figure 3. (c)Use the standard electrode potentials in Table 2 to answer the questions that follow.
(i)Write the half-cell representation for the element whose electrode potential is for hydrogen.
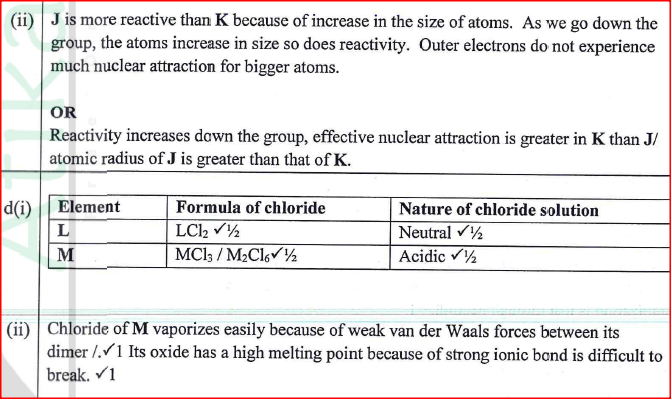
(ii) Arrange the elements in order of reducing power, starting with the weakest reducing agent. (iii) I Select two half cells which combine to give a cell with the least e.m.f. II Calculate the e.m.f of the half cells identified in (iii) I.
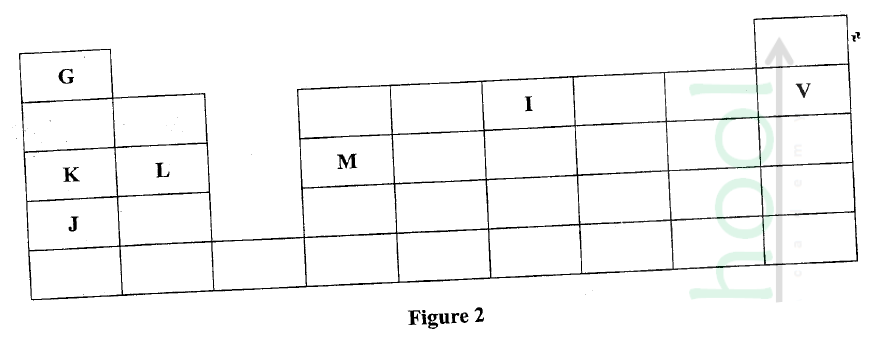
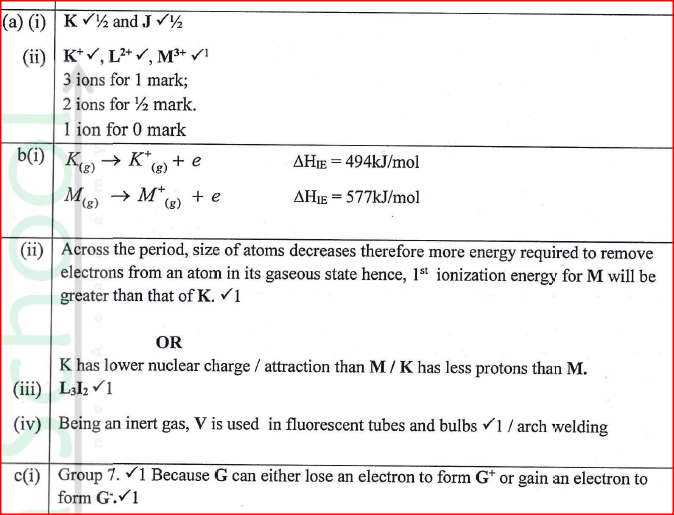
Figure 2 is a section of the periodic table. Study it and answer the questions that follow. The letters do not represent the actual symbols of elements.
(a) (i) Select elements which belong to the same chemical family.
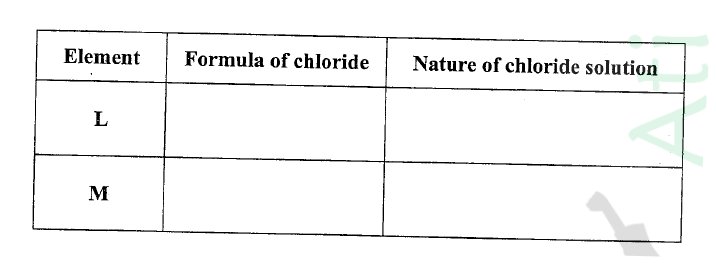
(ii) Write the formulae of ions for elements in the same period. (b) The hrst ionisation energies of two elements K and M at random are 577 kJ/mol and 494 kJ/mol (i) Write equations for the 1ˢᵗ ionisation energies for elements K and M and indicate their energies. (iii) Write the formula of the compound formed when L and I react. (iv) Give one use of elemcnt V. (c) (i) State anothcr group that G can be placcd in Figure 1. Explain. (ii) How do the reactivity of elements J and K compare? Explain. (d) (i) Elements L and M form chlorides. Complete the following table by writing the formulae of each chloride and state the nature of the solutions.
(ii) The chloride of element M vapourises easily while its oxide has a high melting point. Explain.
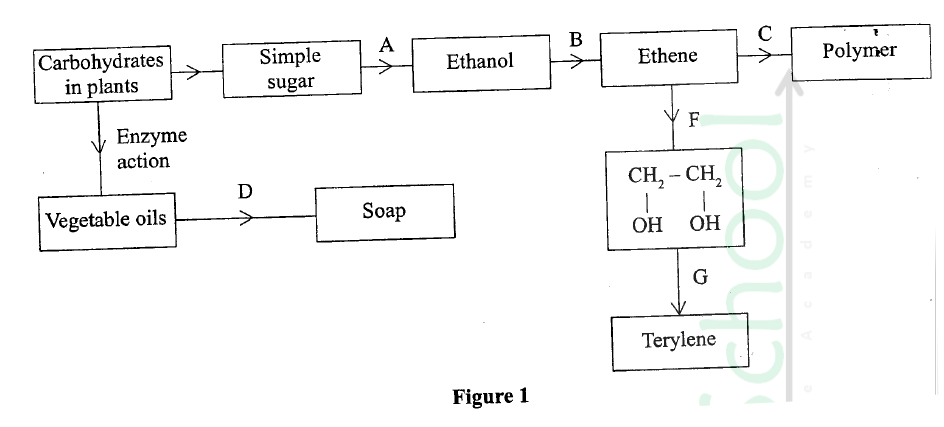
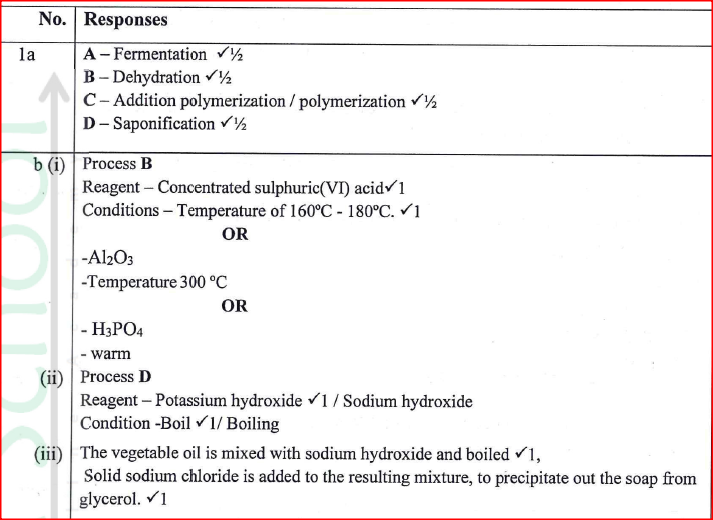
The diagram in figure I shows some natural and industrial processes. Study it and answer the questions that follows
(a) Identify the processes labelled:
A................... B................... C................... D................... (b) State the reagents and conditions required for processes B and D. (i) Process B: Reagent................ Conditions ............ (ii) Process D: Reagent................ Conditions ............ (iii) Describe how process D is carried out. (iv) State two additives used to improve the quality of soap. (c) State the reagents required in steps F and G. (iii) Draw the structure of terylene. (d) (i) Name the polymer formed in step C. (ii) State one disadvantage of the polymer formed in (d) (i).
Distinguish between empirical and molecular formula of a compound.
ANSWERS
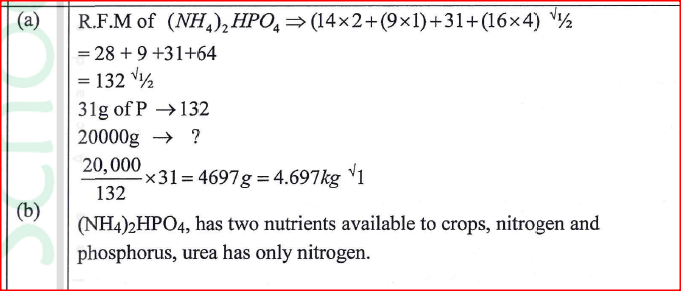
(NH4)2HPO4 is a fertilizer used by farmers to boost their crop production.
(a) Calculate the mass of phosphorus in a 20 kg packet 14.0; H = 1.0; P = 31.0; 0 16.0) (b) State one advantage of this fertilizer, (NH4)2HPO4 over urea CO(NH2)2
Explain why commercial indicators are preferred to flower exacts as acid-base indicators.
ANSWERS
Explain why a solution of sodium chloride Conducts electricity while that of sugar does not.
ANSWERS
(a) Name two ores of iron.
(b) Describe how the amount of iron in a sample of iron(III) oxide can be determined.
ANSWERS
(a) Haematite;
Magnetite; Siderite. (b) Weigh the iron (III) oxide together with a crucible; Heat the Iron(III) oxide and coke to a constant mass; Cool and re-weigh residue and crucible The difference in mass is weight of the iron.
Explain why it is important to put off a non-luminous flame immediately after use.
ANSWERS
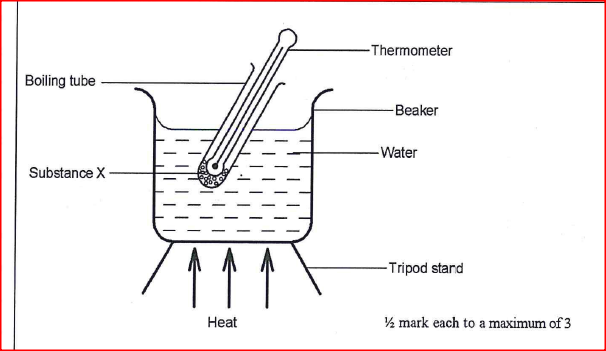
You are provided with the following: thermometer, boiling tube, beaker, Bunsen burner, pure substance X whose boiling point is about 80°C, water and any other apparatus that may be required. Draw a labelled diagram of the set-up that can be used to determine the melting point of X.
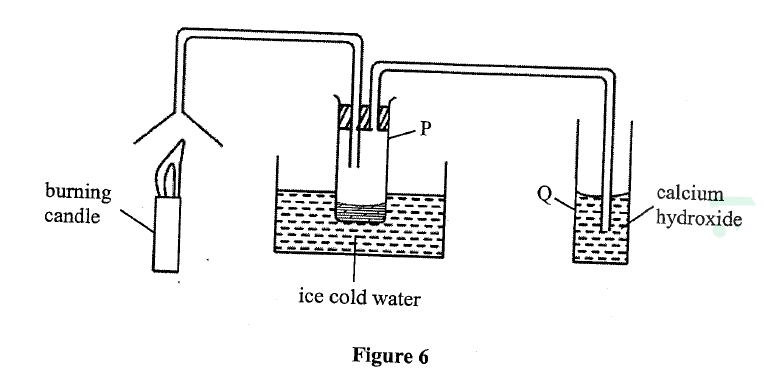
Study the set-up in Figure 6 and answer the questions that follow.
(a) Name the substance that was collected in tube p.
(b) Write an equation for the reaction which occurs in tube Q in the first few minutes of the experiment. (c) Give a suitable Conclusion for the experiment in the set-up.
(a) Zinc reacts with hydrochloric acid according to the following equation.
Identify the reducing agent. Give a reason for the answer.
(b) Iron sheets are dipped in molten zinc to prevent rusting. Name this process.
(a) Give the symbols of the o charged particles emitted by a radioactive isotope.
mass number
atomic number:
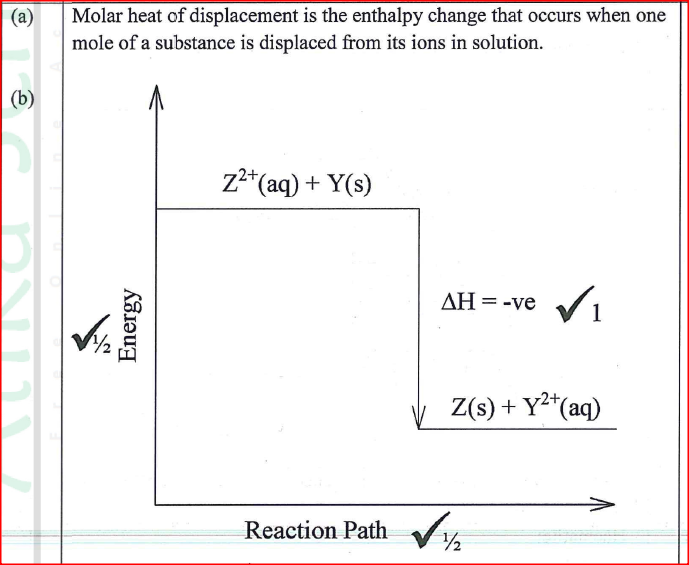
(a) Define molar heat of displacement.
(b)The following ionic equation represents the reaction between metal Y and an aqueous solution of Z2+.
Draw an energy level diagram to represent the reaction
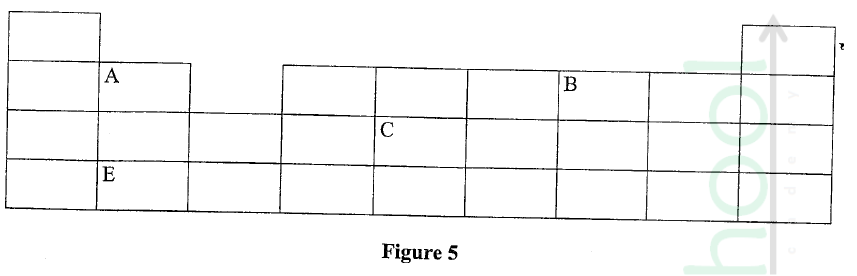
Figure 5 represents a grid that is part of the periodic table. Study it and answer the questions that follow. The letters are not the actual symbols of the elements.
(a) Write the electron arrangement of element C.
(b) On the grid provided, show with a tick (✓) the position of element D whose atomic number is 18. (c) Element E is more reactive than A. Explain.
ANSWERS
(a) 2.8.4
(b) period 3, group 8 (c) E has a bigger atomic radius than A / the valence electrons of element E are further from the nucleus, hence loosely held by the positive nucleus and requires less energy to be removed during reaction. OR A has a smaller atomic radius than E / the valence electrons of element A are closer to the nucleus, hence strongly held by the positive nucleus and requires more energy to be removed during a reaction.
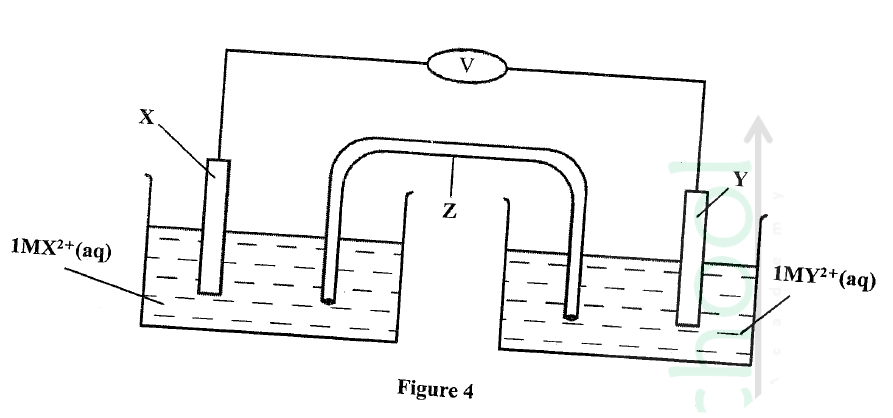
Metal X and Y have standard electrode Potentials of —0.13 V and —0.76V respectively .The metals were Connected to form a cell as shown in Figure 4.
(a) Name the part labelled z.
(b) State one functi0 of the part labelled Z. (c) Calculate the e.m.f. of the cell.
You are provided with solid potassium hydrogen carbonate. Describe how a solid sample of potassium nitrate can be prepared.
ANSWERS
Measure a certain volume of dilute nitric(V) acid and place it in a beaker;
Add potassium hydrogen carbonate little by little as the mixture is stirred until effervescence stops; Evaporate the solution to saturation and allow to cool for crystals to form; Dry the crystals in between filter papers.
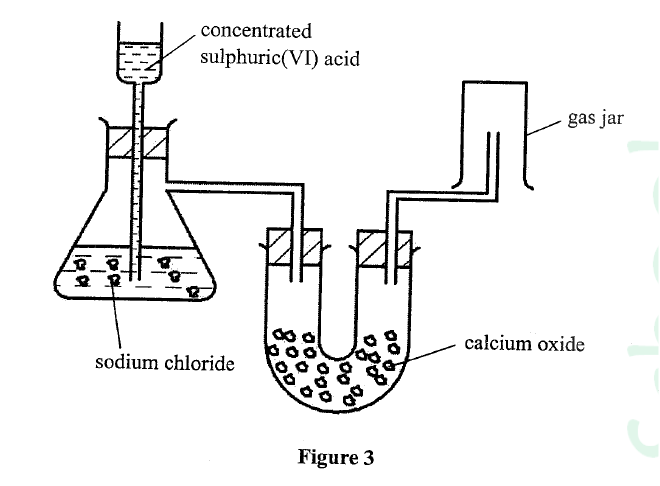
Figure 3 shows a set-up used by a student to prepare dry chlorine gas in the laboratory.
Identify three mistakes in the set-up, and give a reason for each.
ANSWERS
In the Haber process, nitrogen reacts with hydrogen according to the following equation.
(a)What would be the effect of adding a catalyst on the position of the equilibrium?
(b) Explain why it is not advisable to use temperatures higher than 773 K in the Haber process.
ANSWERS
(a)No effect/does not affect the position of the equilibrium.
(b)Forward reaction is exothermic, excessive temperatures would favour the backward reaction therefore lowering the yield of ammonia.
When ethene gas is compressed at a high temperature, a solid is formed.
(a) Give the name of the solid. (b) Explain why it is not advisable to allow the solid to accumulate in the environment.
ANSWERS
(a)Polythene / Polyethene
(b)It is non-biodegradable, hence pollutes the environment.
(a) Element U has atomic number 12 while element V has atomic number 16. How do the melting points of their oxides compare? Explain.
ANSWERS
Using iron filings, describe an experiment that can be conducted to show that oxygen is present in air.
ANSWERS
|
Chemistry Topics
All
Archives
December 2024
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
|
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |

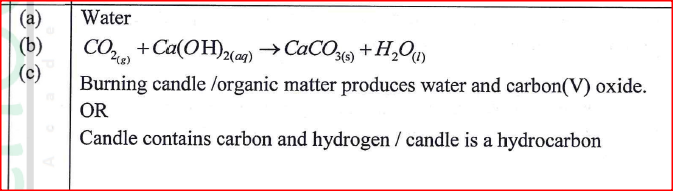


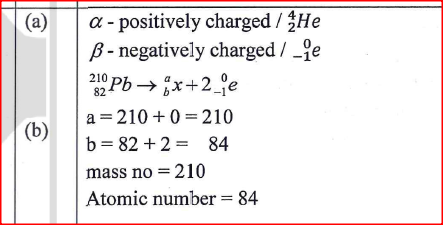
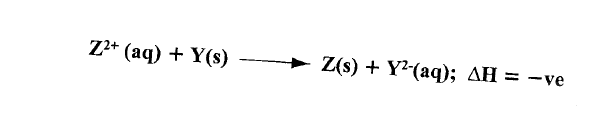

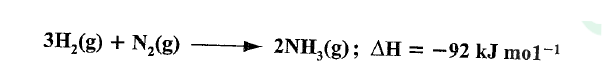

 RSS Feed
RSS Feed

