|
These are chemistry questions and answers categorized according to topics, papers i.e. Paper 1 and 2, Levels i.e. form 1 to form 4, kcse year the examination was done and section A or B
Select topic/category to open topical questions from that particular option provided. Chemistry Topics
0 Comments
Explain why commercial indicators are preferred to flower exacts as acid-base indicators.
ANSWERS
Describe an experiment to show that group one elements react with cold water to form alkaline Solutions
ANSWERS
(a) Define a soluble base.
(b) Aqueous solutions of 2M ethanoic acid and 2M nitric(V) acid were tested for electrical conductivity. Which solution is a better conductor of electricity? Explain.
ANSWERS
(a)A soluble base is a substance that dissociates in water to produce hydroxide ions as the only negative ions.
(b)Nitric(V) acid. This is because nitric(V) acid is a strong acid and dissociates completely in solution producing many H+ ions.
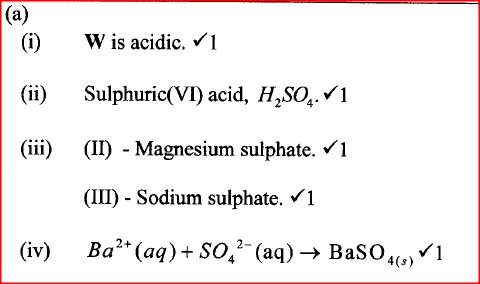
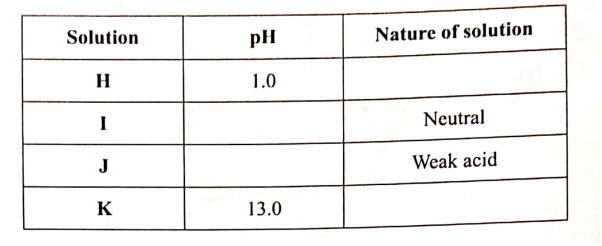
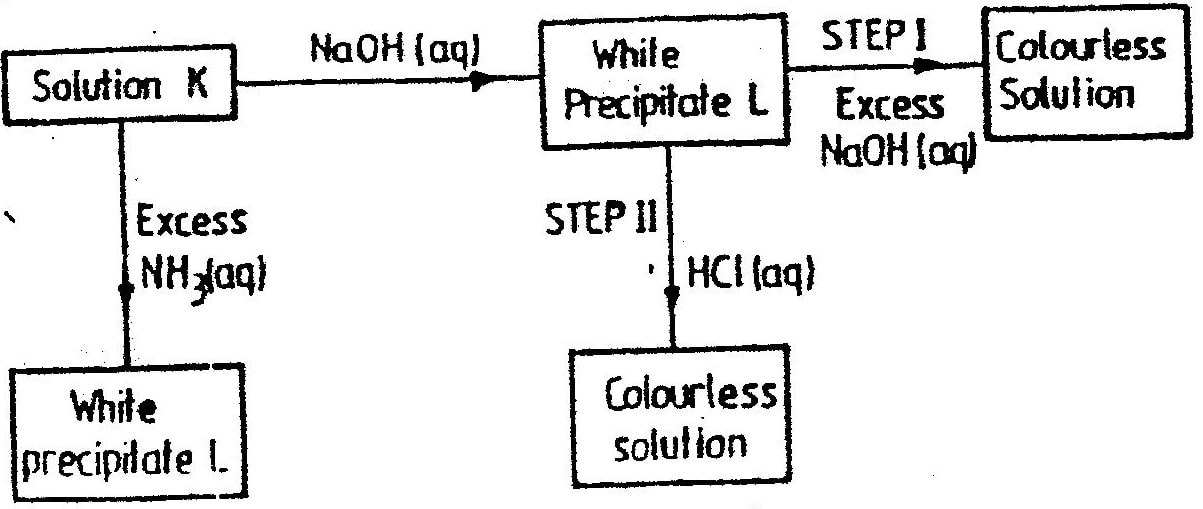
W is a colourless aqueous solution with the following properties:
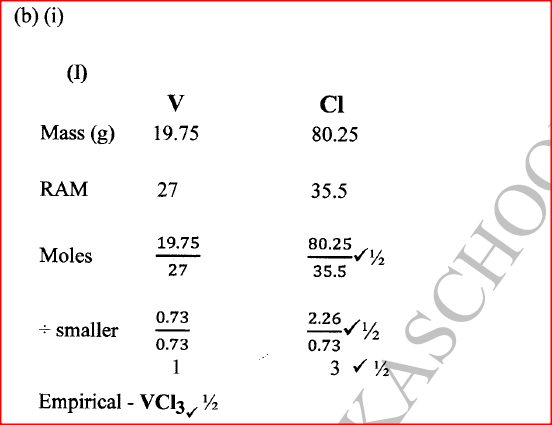
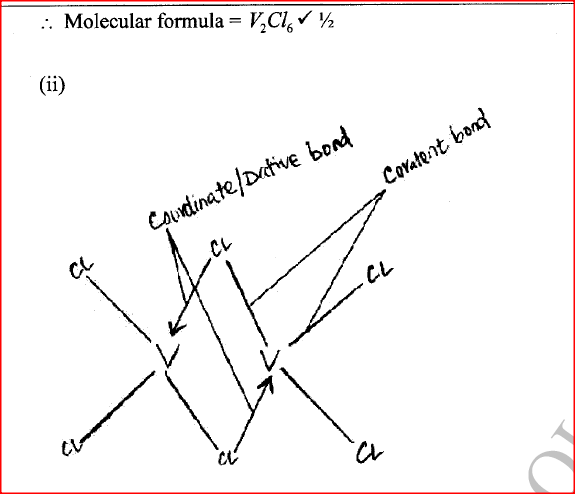
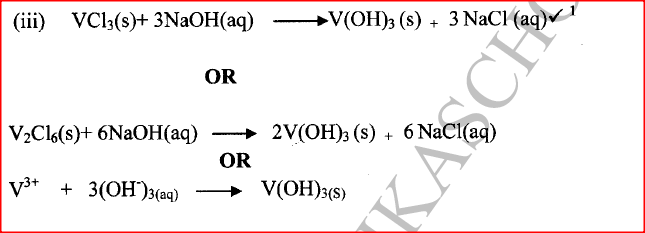
(ii) Give the identity of W. (iii) Name the colourless solution formed in (II) and (III). (iv) Write an ionic equation for the reaction indicated in (V). (b) Element V conducts electricity and melts at 933K. When chlorine gas is passed over heated V, it forms a vapour that solidifies on cooling. The solid chloride dissolves in water to form an acidic solution. The chloride vapour has a relative molecular mass of 267 and contains 19.75% of V. At a higher temperature, it dissociates to a compound of relative molecular mass 133.5. When aqueous sodium hydroxide is added to the aqueous solution of the chloride, a white precipitate is formed which dissolves in excess alkali. (V = 27.0 ; CI = 35.5) (i) Determine the:
(iii) Write an equation for the reaction that form a white precipitate with sodium hydroxide.
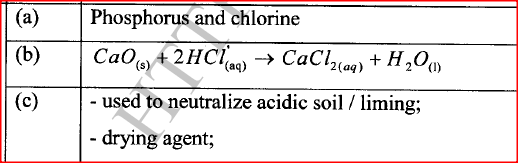
Using the elements chlorine, calcium and phosphorus:
(a) Select elements that will form an oxide whose aqueous solution has a pH less than 7. (b) Write an equation for the reaction between calcium oxide and dilute hydrochloric acid. (c) Give one use of calcium oxide.
Given the following substances: wood ash, lemon juice and sodium chloride.
(a) Name one commercial indicator that can be used to show whether wood ash, lemon juice and sodium chloride are acidic, basic or neutral. (b) Classify the substances in 15(a) above as acids, bases or neutral.
ANSWERS
(a) Litmus
Phenolphthalein indicator (b) Wood ash- Basic Lemon Juice - acidic Sodium Chloride- neutral
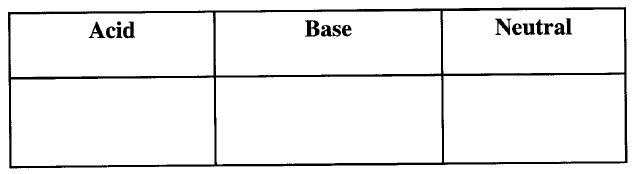
(a)Complete the following table. (2 marks)
(b) Explain why a solution of ammonia in methylbenzene has no effects on red litmus paper while in aqueous ammonia red litmus paper turns blue. (1 mark)
Given the following substances: wood ash, lemon juice and sodium chloride.
(a) Name one commercial indicator that can be used to show whether wood ash, lemon juice and sodium chloride are acidic, basic or neutral. b) Classify the substances in 15(a) above as acids, bases or neutral
ANSWERS
(a) Universal indicator / litmus paper
(b) Acid, base, neutral.
A farmer intended to plant cabbages in his farm. He first tested the pH of the soil and found it to be 3.0. If cabbages do well in alkaline soils, explain the advice that would be given to the farmer in order to realise a high yield. (2 marks)
a) Name a suitable solvent for extracting an indicator form flowers:
b) Give a reason why the solvent named in (a) above is used
ANSWERS
(a) Acetone / ethanol / propanone / propanol.
(b) The solvent dissolves the organic compound indicator present in the flowers / it is an organic solvent.
The Ph of a sample of soil was found to be 5.0.An agricultural office recommended the addition of calcium oxide in the soil. State two functions of the calcium oxide in the soil.
Expected Response
10gm of sodium hydrogen carbonate were dissolved in 20cm3 of water in a boiling tube. Lemon juice was then added drop wise with shaking until there was no further observable change.
(a) A student was supplied with a colourless liquid suspected to be water
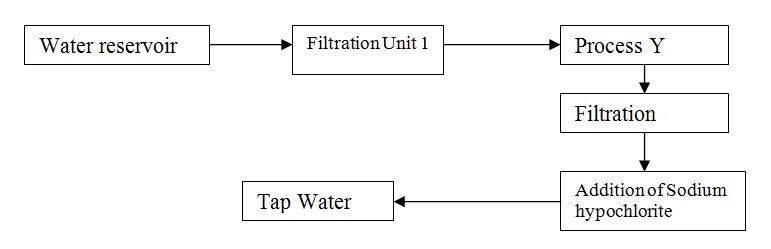
The flow chart below shows the various stages of water treatment. Study it and answer the questions that follow
II Addition of sodium hypochlorite (c) It was confirmed that magnesium sulphate was present in the tap water
Starting with copper metal, describe how a solid samples of copper (II) carbonate can be prepared.
Describe how the PH of anti-acid (Actal) powder can be determined in the laboratory
ANSWERS
ANSWERS
When a student was stung by a nettle plant, a teacher applied an aqueous solution of ammonia to the affected area of the skin and the student was relieved of pain .Explain.
ANSWERS
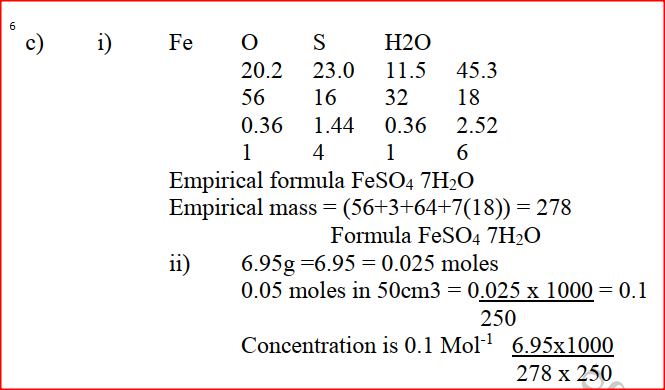
a) Give the name of each of the processes described below which takes place when salts are exposed to air for sometime. i) Anhydrous copper sulphate becomes wet (1mk) ii) Magnesium chloride forms an aqueous solution (1mk) iii) Fresh crystals of sodium carbonate, Na2CO3. 10H2O (1mk) b) Write the formula of the complex ion formed in each of the reactions described below. (i) Zinc metal dissolves in hot alkaline solution (1mk) (ii) Copper hydroxide dissolves in excess ammonia solution. (1 mk) (c) A hydrated salt has the following composition by mass. Iron 20.2% Oxygen 23.0%, sulphur 11.5%, water 45.3 %. Its relative formula mass is 278. (i) Determine the formula of the hydrated salt.. (3mks) (Fe=56, S=32; O = 16, H =1) (ii) 6.95gm of the hydrates salt were dissolved in distilled water and the total volume made to 250 cm3 of solution. Calculate the concentration of the salt solution in moles per litre. Explain how you would obtain solid carbonate from a mixture of lead carbonate and sodium carbonate powders. (3mks)
Expected Response
Dissolve in water, filter to remove lead carbonate as a residue, evaporate filter to saturation and allow to cool. Crystallization to take place. Filter the crystals and dry. Evaporate to dryness
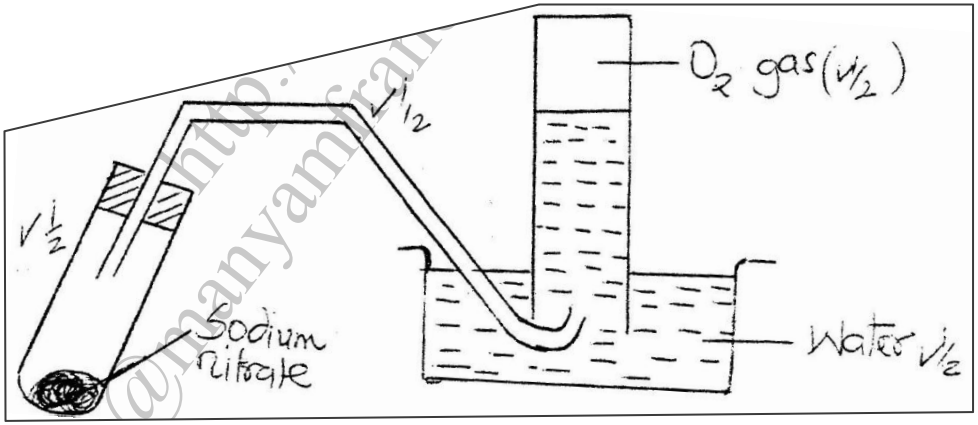
On strong heating, sodium nitrate oxygen gas. In the spaces provided below, draw a labeled diagram of a set-up that could be used for heating sodium nitrate and collecting the oxygen gas liberated. (3mks)
(a) what method can be used to separate a mixture of ethanol and propanol?
(b) (i) Explain how a solid mixture of sulphur and sodium chloride can be separated Into solid sulphur and solid chloride (c) The table below gives the solubilities of potassium bromide and potassium sulphate at 00C and 400C
When an aqueous mixture containing 60g of potassium and 7 g of potassium sulphate in 10g of water at 800C was cooled to 00C some crystals were formed
(i) Identify the crystals (ii) Determine the mass of the crystals formed (iii) Name the method used to obtain the crystals (iv) Suggest one industrial application of the method named in (iii) above
The following tests were carried out on three separate portions of a colourless solution S
(a) From the information in test (i), name a cation, which is not present in solution S.
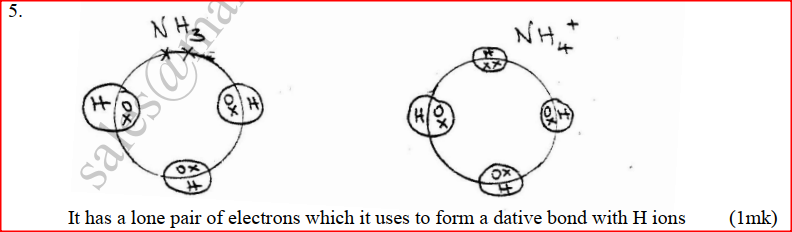
(b) Identify a cation, which is likely to be present in solution S (c) Write an ionic equation for the reaction, which takes place in test (ii) a) Using dots(.) and crosses (x) to represent electrons draw diagram to represent the bonding in: (i) NH3 (ii) NH4+ (1mk) b) State why an ammonia molecule (NH3) can combine with H+ to form NH4+ (Atomic numbers: N=7 and H=1) (1mk) |
Chemistry Topics
All
Archives
December 2024
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
|
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |
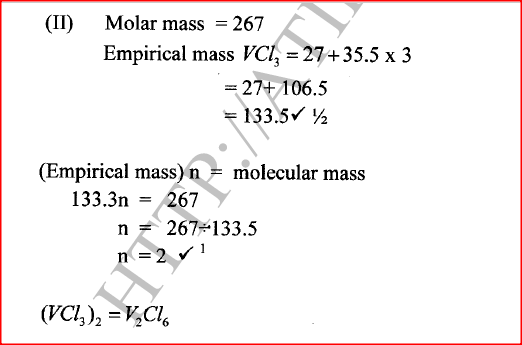








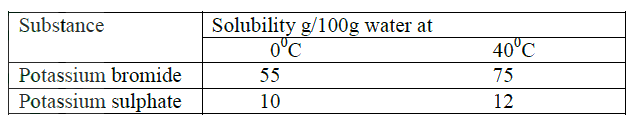


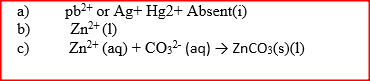

 RSS Feed
RSS Feed

