|
These are chemistry questions and answers categorized according to topics, papers i.e. Paper 1 and 2, Levels i.e. form 1 to form 4, kcse year the examination was done and section A or B
Select topic/category to open topical questions from that particular option provided. Chemistry Topics
0 Comments
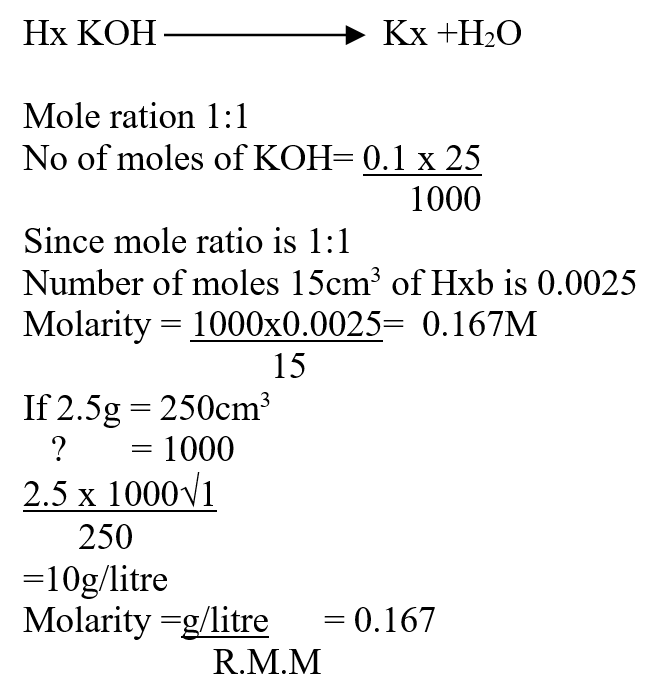
a) The solubility of the salt (2mks)b) The percentage of the salt in the saturated solution (1mk)A mass of 2.5g of acid HX was dissolved in water and the resulting solution was diluted to a total of 250cm3, 15cm3 of the final solution was required to neutralize 25.0cm3 of 0.1M aqueous potassium hydroxide. Calculate the relative molecular mass of the acid (3mks)a) Sugar crystals. (1mk)
b) Hydrated copper (II) sulphate solution (1mk)
c) What type of reaction has taken place above (1mk)
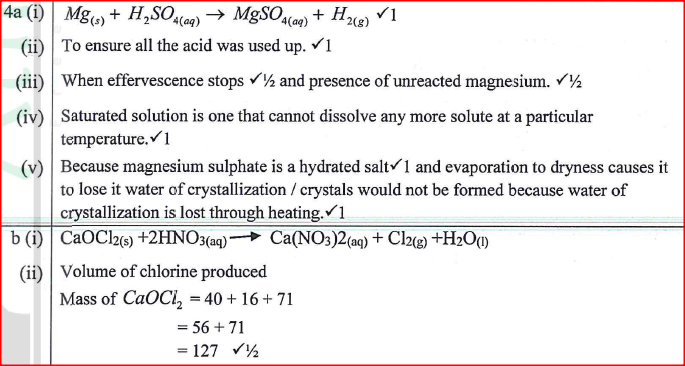
An experiment was carried out to prepare crystals of magnesium sulphate. Excess magnesium powder was added to 100 cm3 of dilute sulphuric (VI) acid in a beaker and warmed until no further reaction took place. The mixture was filtered and the filtrate evaporated to saturation, then left to cool for crystals to form.
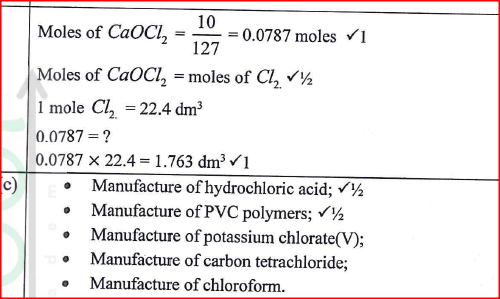
(a) (i) Write an equation for the reaction. (ii) Explain why excess magnesium powder was used. (iii) State how completion of the reaction was determined. (iv) What is meant by a saturated solution? (v) Explain why the filtrate was not evaporated to dryness. (b) When bleaching powder, CaOCl2, is treated with dilute nitric(V) acid, chlorine gas is released. This reaction can be used to determine the chlorine content of various samples of bleaching powders and liquids. (i) Write an equation for the reaction of nitric(V) acid with bleaching powder. (ii) Calculate the volume of chlorine produced when 10g of CaOCl2 is treated with excess nitric (v) acid. (Ca = 40.0; 0 = 16.0; Cl = 35.5; 1 mole of gas occupies 22.4dm3 at s.t.p) (c) Apart from use of chlorine gas in bleaches and water treatment, state two other uses of chlorine gas.
You are provided with solid potassium hydrogen carbonate. Describe how a solid sample of potassium nitrate can be prepared.
ANSWERS
Measure a certain volume of dilute nitric(V) acid and place it in a beaker;
Add potassium hydrogen carbonate little by little as the mixture is stirred until effervescence stops; Evaporate the solution to saturation and allow to cool for crystals to form; Dry the crystals in between filter papers.
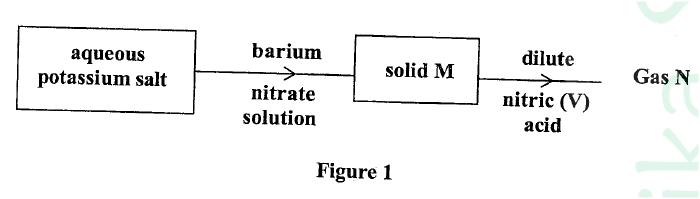
Study the flow chart in Figure 1 and answer the questions that follow.
Gas N forms a while suspension with aqueous calcium hydroxide.
(a) Name the anion present in the potassium salt. (b) Write an ionic equation for the formation of solid M. (c) Give one use of gas N.
(a) Define a soluble base.
(b) Aqueous solutions of 2M ethanoic acid and 2M nitric(V) acid were tested for electrical conductivity. Which solution is a better conductor of electricity? Explain.
ANSWERS
(a)A soluble base is a substance that dissociates in water to produce hydroxide ions as the only negative ions.
(b)Nitric(V) acid. This is because nitric(V) acid is a strong acid and dissociates completely in solution producing many H+ ions.
The following steps were used to analyse a metal ore.
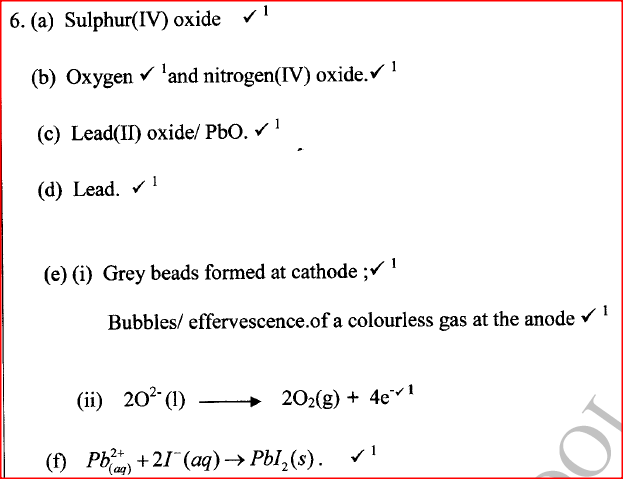
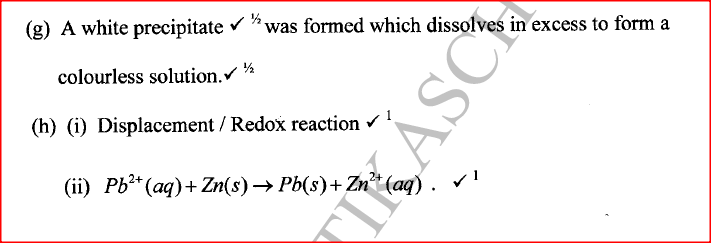
(i) An ore of a metal was roasted in a stream of oxygen. A gas with a pungent smell was formed which turned acidified potassium dichromate(VI) green. (ii) The residue left after roasting was dissolved in hot dilute nitric(V) acid. Crystals were obtained from the solution. (iii) Some crystals were dried and heated. A brown acidic gas and a colourless gas were evolved and a yellow solid remained. (iv) The solid was yellow when cold. (v) The yellow solid was heated with powered charcoal. Shiny beads were formed. Name the: (a) gas formed when the ore was roasted in air. (b) gases evolved when crystals in step (iii) were heated. (c) yellow solid formed in step (iii). (d) shiny beads in step (iv). (e) The yellow solid from procedure (iii) was separated, dried, melted and the melt electrolysed using graphite electrodes. I. Describe the observations made at each electrode. II. Write the equation for the reaction that took place at the anode. (f) Some crystals formed in step (ii) were dissolved in water, and a portion of it reacted with potassium iodide solution. A yellow precipitate was formed. Write an ionic equation for this reaction. (g) To another portion of the solution from (f), sodium hydroxide solution was added drop by drop until there was no further change. Describe the observation made. (h) To a further portion of the solution from (f), a piece of zinc foil was added. I. Name the type of reaction taking place. II. Write an ionic equation for the above reaction.
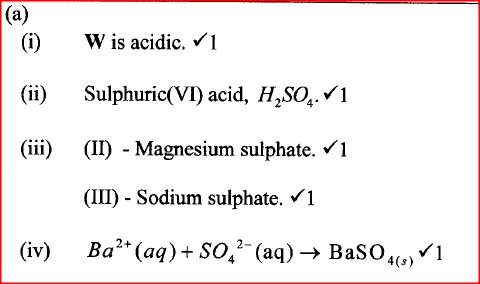
W is a colourless aqueous solution with the following properties:
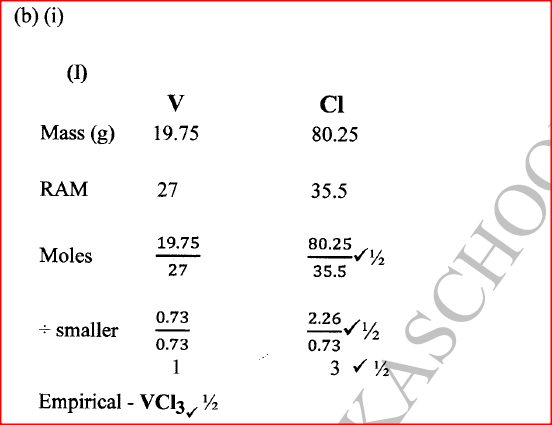
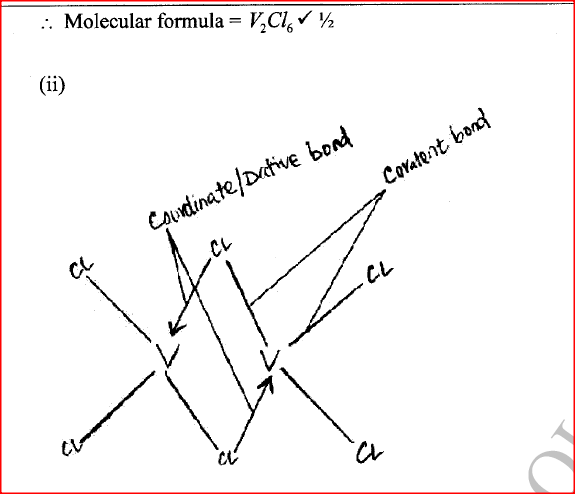
(ii) Give the identity of W. (iii) Name the colourless solution formed in (II) and (III). (iv) Write an ionic equation for the reaction indicated in (V). (b) Element V conducts electricity and melts at 933K. When chlorine gas is passed over heated V, it forms a vapour that solidifies on cooling. The solid chloride dissolves in water to form an acidic solution. The chloride vapour has a relative molecular mass of 267 and contains 19.75% of V. At a higher temperature, it dissociates to a compound of relative molecular mass 133.5. When aqueous sodium hydroxide is added to the aqueous solution of the chloride, a white precipitate is formed which dissolves in excess alkali. (V = 27.0 ; CI = 35.5) (i) Determine the:
(iii) Write an equation for the reaction that form a white precipitate with sodium hydroxide.
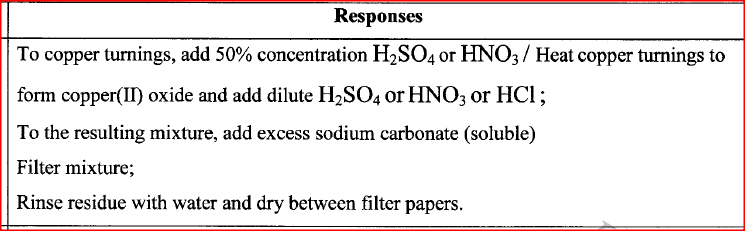
Starting with copper, describe how a pure sample of copper(II) carbonate can be prepared.
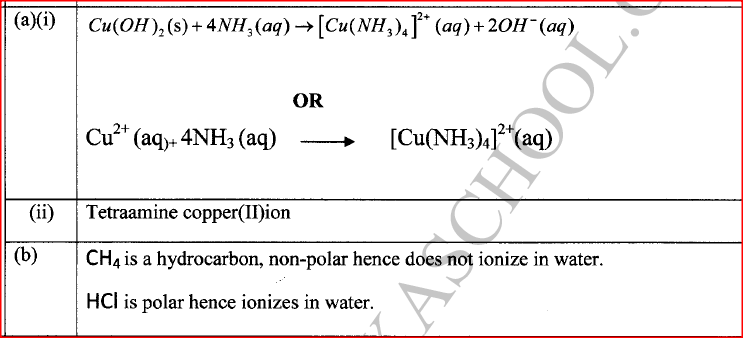
Copper(II) ions react with excess aqueous ammonia to form a complex ion.
(a) (i) Write an equation for the reaction that forms the complex ion. (ii) Name the complex ion. (b) Explain why CH4 is not acidic while HCl is acidic yet both compounds contain hydrogen.
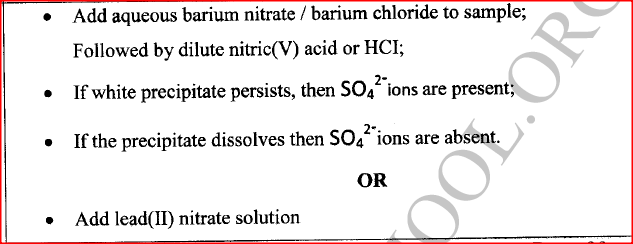
A sample of water is suspected to contain sulphate ions. Describe an experiment that can be carried out to determine the presence of sulphate ions.
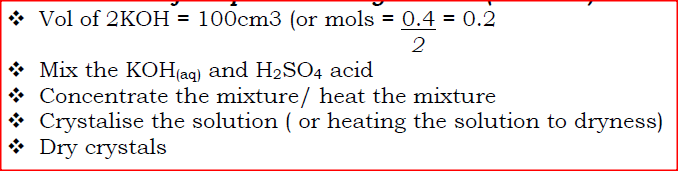
Describe how a solid sample of potassium sulphate can be prepared starting with 200cm3of 2M potassium hydroxide.
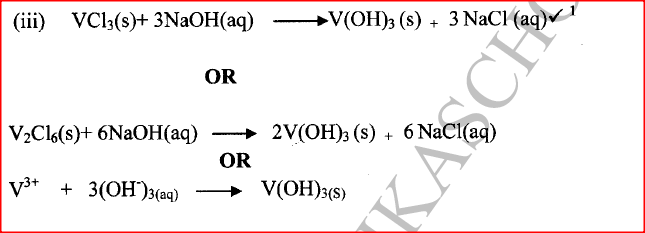
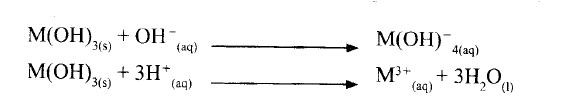
A compound whose general formula is M(OH)3 reacts as shown by the equation below.
(a) What name is given to compounds which behave like M(OH) 3 in the two reactions.
(b) Name two elements whose hydroxides behave like that of M.
ANSWERS
(a) Amphoteric
(b)Lead, Zinc and Aluminium
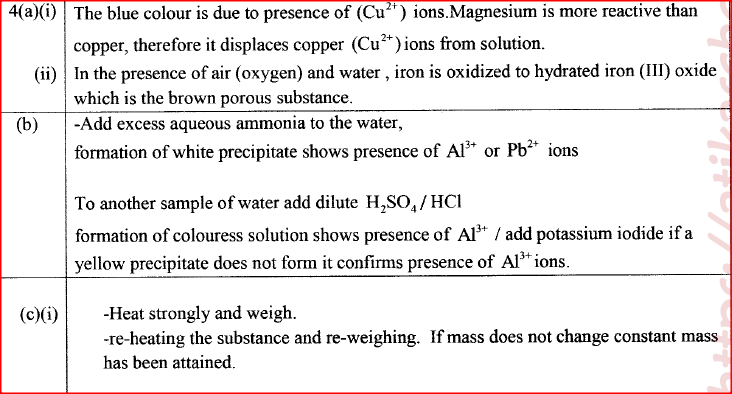
(a) Explain the following observations:
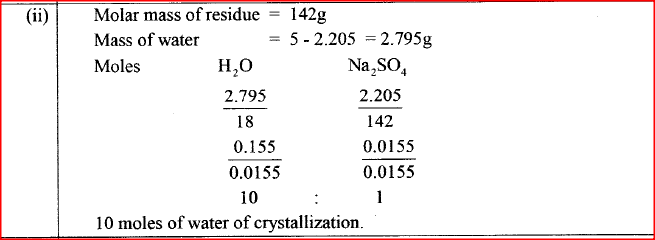
(b) A sample of water is suspected to contain aluminium ions (AI3+) . Describe a laboratory experiment that can be carried out to show that AI3+ ions are present in the water sample. (3 marks) (c) In an experiment to determine the number of moles of water of crystallisation of a hydrated compound, Na2S04. X H2O, 5g of the compound were heated strongly to a constant mass.
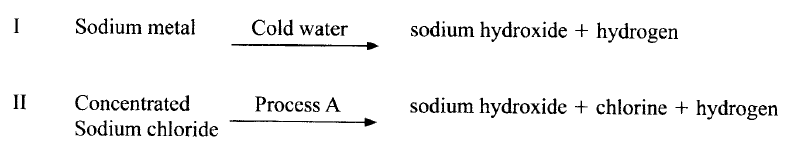
Sodium hydroxide can be prepared by the following methods; I and II
(a) Name one precaution that needs to be taken in method I.
(b) Give the name of process A. (c) Give one use of sodium hydroxide.
ANSWERS
(a) Small piece of sodium metal (pea size) with a lot of water
Perform the experiment wearing goggles. (b) Electrolysis (c) Manufacture of paper (soften), soaps and detergents Fractional distillation of liquid air Extraction of aluminium metal Manufacture of bleaching agents eg NaOCl paper, textiles, oil refinery Making herbicides on weed killers Textile industry to soften
Starting with sodium metal, describe how a sample of crystals of sodium hydrogen carbonate may be prepared.
ANSWERS
React sodium with water to get sodium hydroxide. Bubble into this solution excess carbon (IV) oxide to get sodium hydrogen carbonate
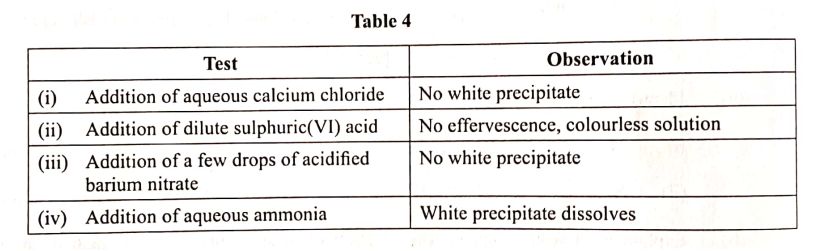
Chemical tests were carried out on separate samples of water drawn from the same source. The observations made were recorded as shown in Table 4.
State the inferences made in reactions:
(a) Name the method that can be used to obtain pure iron (III) chloride from a mixture of iron (III) chloride and sodium chloride.
(b) A student was provided with a mixture of sunflower flour, common salt and a red dye. The characteristics of the three substances in the mixture are given in the table below.
The student was provided with ethanol and any other materials needed.
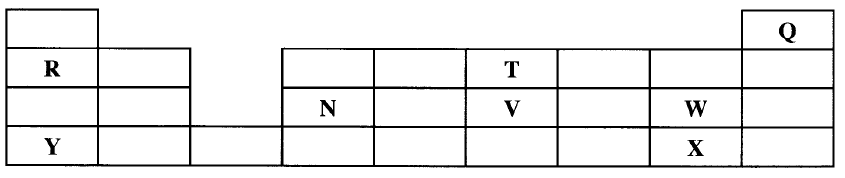
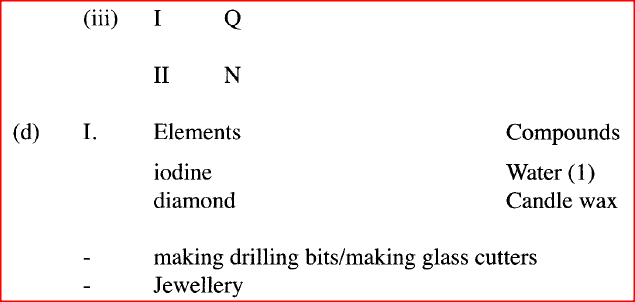
Described how the student can separate the mixture into its three components c) The diagram below show part of a periodic table. The letters do no represent the actual symbols of elements. Use the diagram to answer the questions that follow.
i) Explain why the oxidizing power of W is more than that of X
ii) How do the melting points of R and T compare? Explain iii) Sketch an element that could be used i) In weather ballons ii) For making a cooking pot d i) Classify the substances water, iodine, diamond and candle wax into elements and compounds
ii) Give one use of diamond
ANSWERS
(a)Sublimation
(b)Add ethanol to the mixture . Filter and evaporate filtrate to obtain red dye . Add water to the residue . Filter to obtain sunflower flour . Evaporate filtrate to obtain salt . OR Add H,O to mixture , filter , residue is sunflower , evaporate the water ; add ethanol to the residue filter . The filtrate is red dye. (3 marks) (c)(i) W accepts electrons more readily than X. W has small atomic radius/ W has less energy levels than X/ W has less screening effect than X/ W has greater effective nuclear attraction than X. W is more electro negative than X. (ii) T has a lower melting point than R because it exists in simple molecular form with weak Van der Waals forces while R has strong metallic bonds.
Starting with barium nitrate solution, describe how a pure sample of barium carbonate can be prepared in the laboratory.
ANSWERS
Describe how samples of lead (II) sulphate, ammonium chloride and sodium chloride can be obtained from a mixture of the three.
ANSWERS
Hydrogen chloride gas can be prepared by reacting sodium chloride with an acid.
(a) Write an equation for the reaction between sodium chloride and the acid. (b) Give two chemical properties of hydrogen chloride gas (c) State two uses of hydrogen chloride
Describe how sodium carbonate is used to remove water hardness
a) Dissolving of potassium nitrate in water is an endothermic process. Explain the effect of increase in temperature on the solubility of potassium nitrate
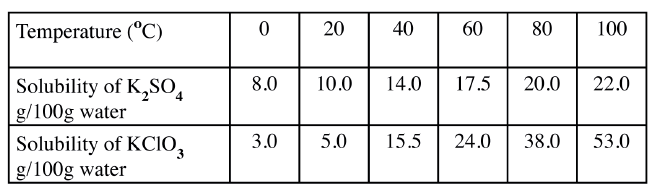
b) The table below shows the solubilities of potassium sulphate and potassium chlorate (V) at different temperatures.
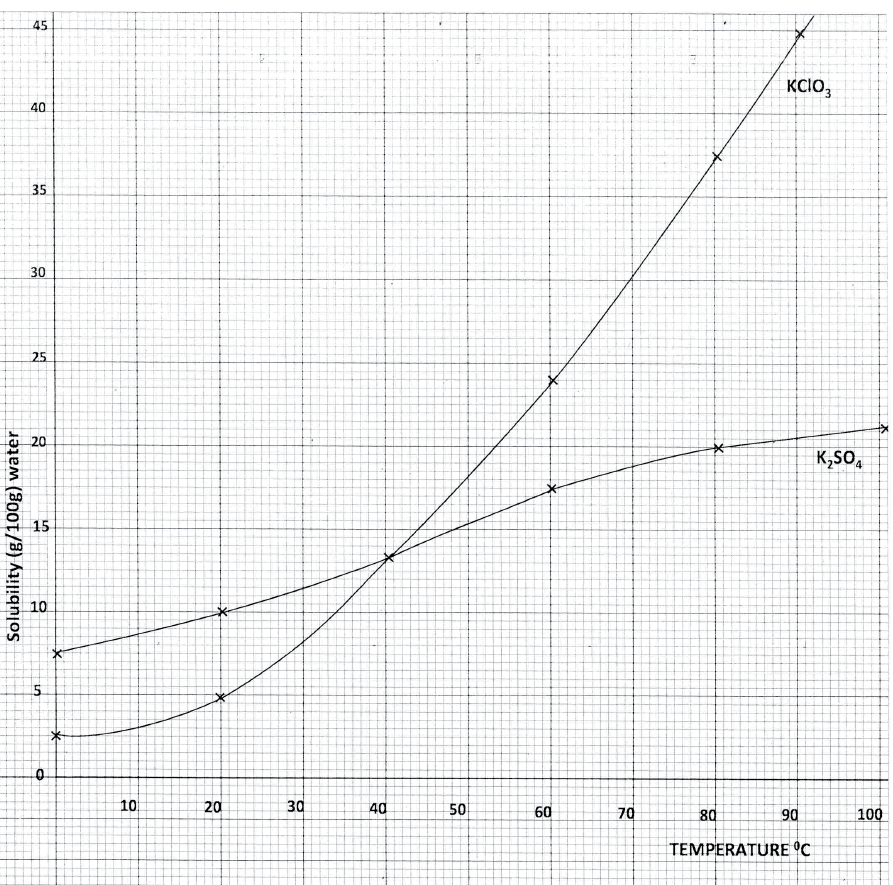
i) Draw the solubility curves for both salts on the same axis. (Temperature on the X-axis
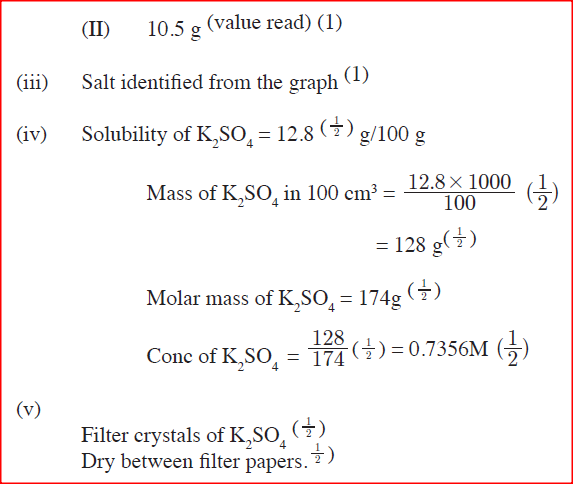
ii)A solution of potassium sulphate contains 20g of the salt dissolved in 100 g of water at 100°C. This solution is allowed to cool to 25°C I) at what temperature will crystals first appears? II) What mass of crystals will be present at 25°C? iii) Which of the two salts is more soluble at 30°C? iv) Determine the concentration of potassium sulphate in moles per litre when the solubility of the two salts are the same (K= 39.0, O=16.0 ; S=32.0) v) 100 g of water at 100°C contains 19g of potassium sulphate and 19 g of potassium chlorate (V). Describe how a solid sample of potassium sulphate at 60°C can be obtained |
Chemistry Topics
All
Archives
December 2024
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
|
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |
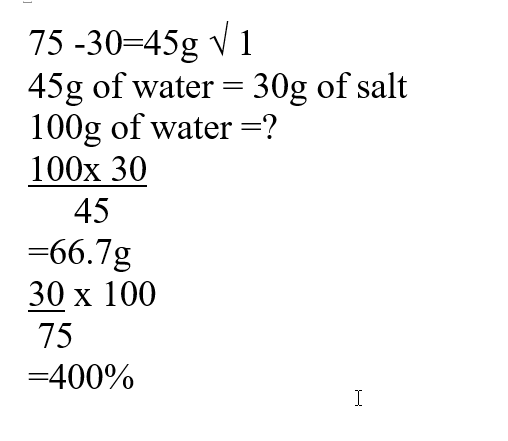


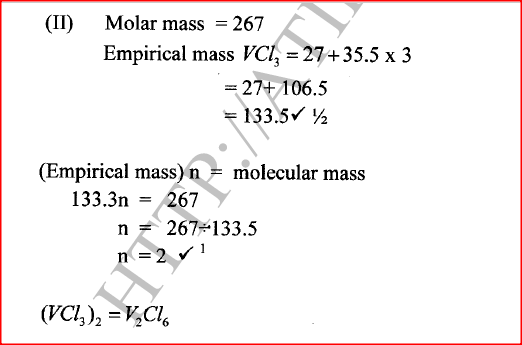




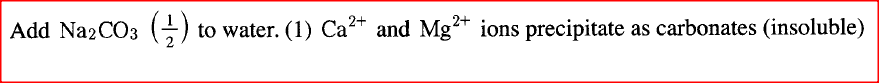

 RSS Feed
RSS Feed

