|
These are chemistry questions and answers categorized according to topics, papers i.e. Paper 1 and 2, Levels i.e. form 1 to form 4, kcse year the examination was done and section A or B
Select topic/category to open topical questions from that particular option provided. Chemistry Topics
0 Comments
Define oxidation and reduction in terms of electrons
Oxidation is the loss of electrons while reduction is gain electrons
Calculate the oxidation number of Chromium in Cr2O2- (1mk)
x * 2+(-2)=-2
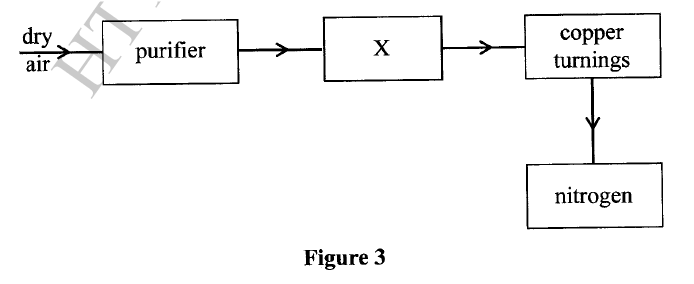
The flow chart in Figure 3 shows the process of obtaining a sample of nitrogen gas. Study it and answer the questions that follow.
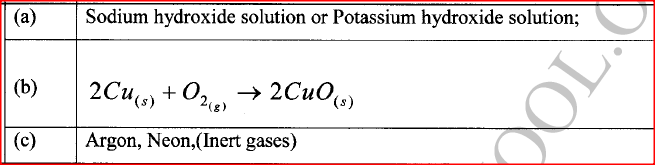
(a) Identify X
(b) Write an equation for the reaction with heated copper turnings. (c) Name an impurity in the sample of nitrogen gas.
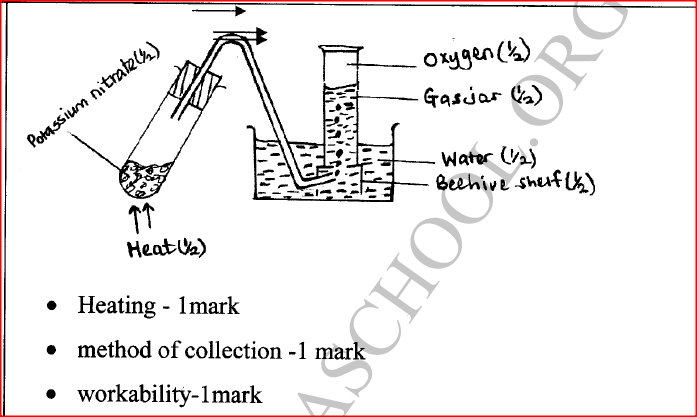
Potassium nitrate liberates oxygen gas when heated. Draw a diagram of a set-up that shows heating of potassium nitrate and collection of oxygen gas.
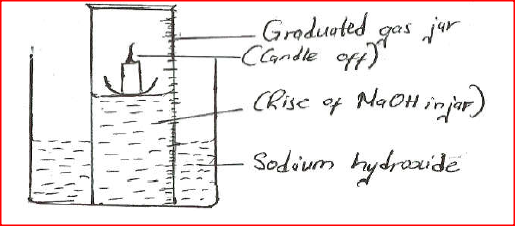
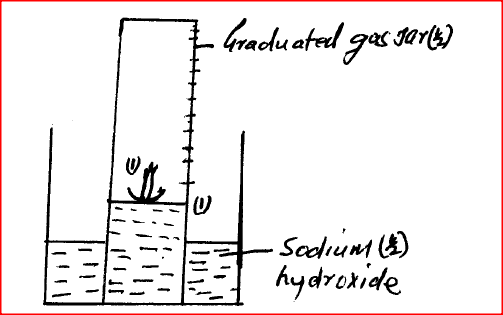
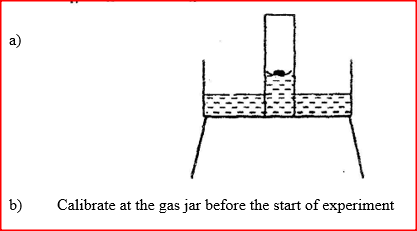
A water trough, aqueous sodium hydroxide, burning candle, watch class and a graduated gas jar were used in an experimental set up to determine the percentage of active part of air. Draw a labeled diagram of the set up at the end of the experiment.
When solid A was heated strongly, it gave off water and a solid residue. When water was added to the solid residue, the original solid A, was formed
(a) What name is given to the process described? (b) Give one example of solid A
ANSWERS
(a)Type of reaction: Reversible reaction/temporary reaction.
(b)Copper (II) Sulphate salt (Crystals) Copper (II) Chloride hydrated. Any other hydrated salts e.g. Cobalt (II) Chloride
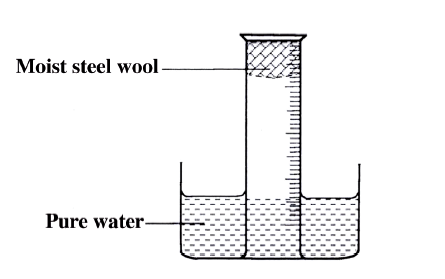
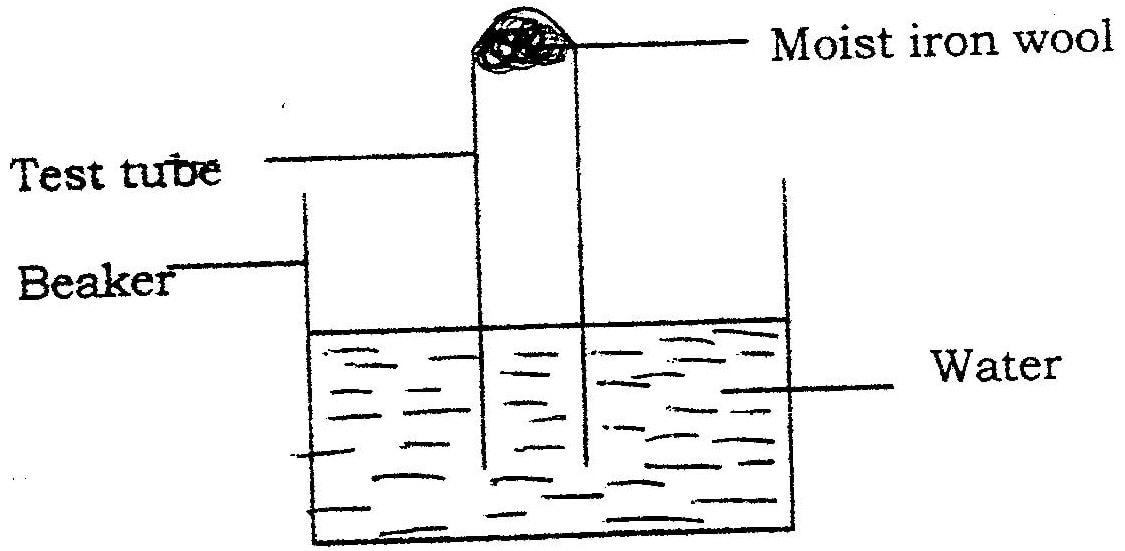
A measuring cylinder fitted with moist steel wool was inverted in a trough of water as shown in the diagram below
(a)State and explain the observations made on the;
i) Moist steel wool after four days. ii) Water level in the measuring cylinder after four days. (b) What would be the effect of using steel wool moistened with salty water?
ANSWERS
(a) (i) It turned brown /blue/violet/green.
(ii) The water level rose up the gas jar/occupy space left by reacted 02 (b) The brown colour would be more since the salt accelerates rusting/rust faster.
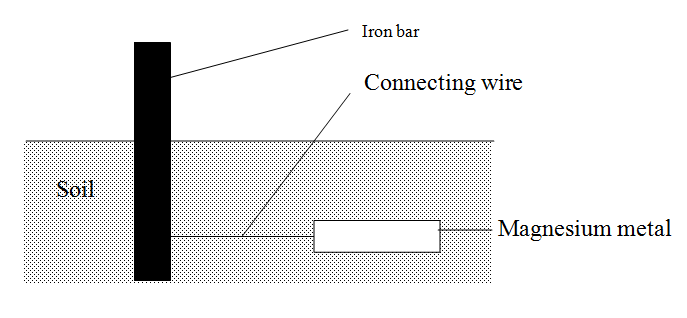
The diagram below shows an iron bar, which supports a bridge. The Iron bar is connected to a piece of magnesium metal
Explain why it is necessary to connect the piece of magnesium metal to the iron bar.
Expected Response
Magnesium is above iron in the activity series. It supplies electrons to the iron bar Hence prevent it from rusting
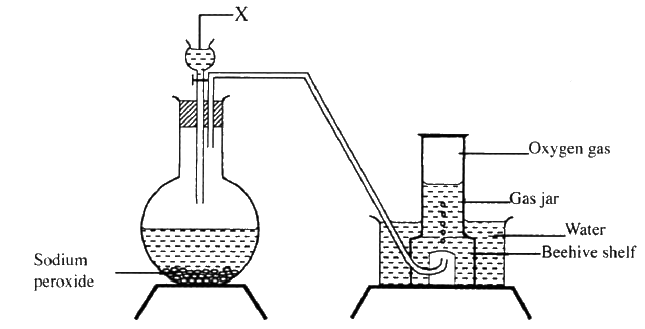
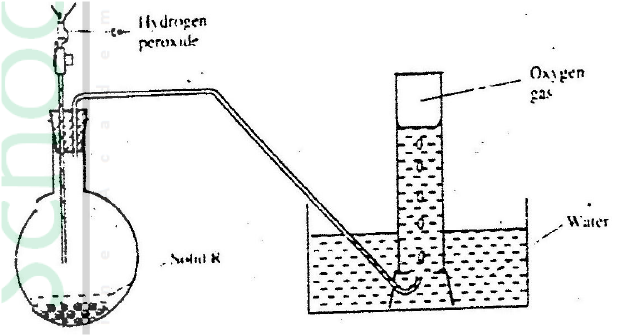
The set up below can be used to prepare oxygen gas. Study it and answer the questions that follow.
(a) Identify X
(b) What property of oxygen makes it possible for it to be collected as shown in the above set up? (c) State two uses of oxygen
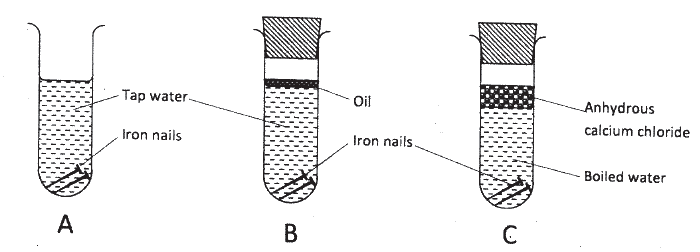
The following set up of three test-tubes was used to investigate rusting of iron.
Study it and answer the questions that follow.
(a) Give a reason why rusting did not occur in test-tube C.
(b) Aluminium is used to protect iron sheets from rusting. Explain two ways in which aluminium protects iron from rusting.
ANSWERS
(a) No air due to boiling.
(b) Aluminium being very reactive forms a layer of Al2O3 on the metal making it impervious to moisture. Aluminium being more reactive than iron protects the iron through sacrificial protection cathodic protection.
Name another gas, which is used together with oxygen in welding
Expected Response
Acet5ylene (ethyne) or Hydrogen
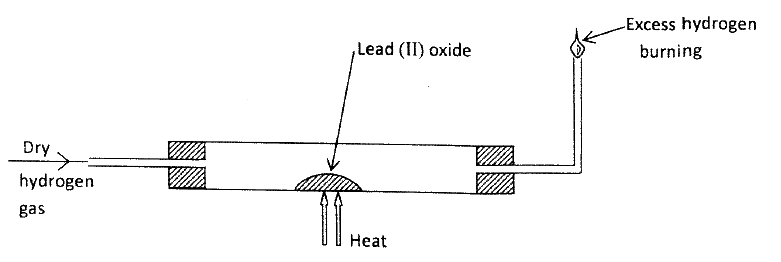
In an experiment, dry hydrogen gas was passed over heated Lead (II) Oxide as shown in the diagram below.
State and explain the observations made in the combustion tube.
ANSWERS
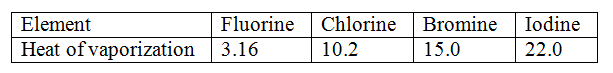
Use the information in the table below to answer the questions that follows
Explain the trend in the molar heats of vaporization
Expected Response
Change was greatest with magnesium. Both react with oxygen to form oxides but magnesium reacts with nitrogen to form a nitrite
A water trough, aqueous sodium hydroxide, burning candle, watch class and a graduated gas jar were used in an experimental set up to determine the percentage
of active part of air. Draw a labeled diagram of the set up at the end of the experiment.
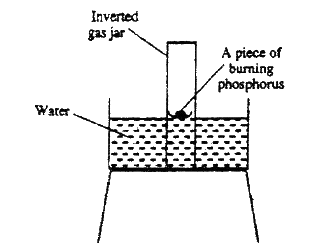
The diagram below represent a set-up that was used to show that part of air is used during burning.
a) Given that phosphorus used was was in excess, draw a diagram of the set-up at the end of the experiment (when there was no further observable change).
b) Suggest one modification that should be made on the apparatus if the percentage of the air used is to be determined.
Expected Response
- Iron wool turns or rusts due to formation of hydrated iron (III) oxide
- Level of water inside the tube rises to occupy the space left by oxygen - Level of water in the beaker will fall
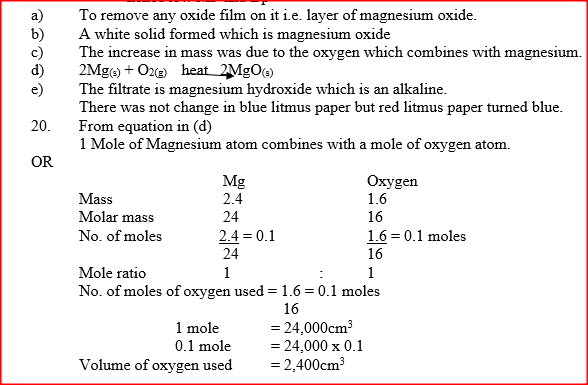
In an experiment, a piece of magnesium ribbon was cleaned with steel wool. 2.4 g of the clean magnesium ribbon was placed in a crucible and completely burnt in oxygen. After cooling, the product weighed 4.0 g
(a) Explain why it was necessary to clean the magnesium ribbon (b) What observation was made in the crucible after burning (c) Why was there an increase in mass? (d) Write the equation for the reaction which took place in the crucible (e) The product in the crucible was shaken with water and filtered. Explain the observation which was made when blue and red litmus papers were dropped into the filtrate.
Write an equation for the reaction that takes place when carbon monoxide gas is passed over heated lead (II) oxide. (1mks)
When wood is burnt, a grey powder called ash remains. The ash is stirred with water and filtered, a colourless solution is obtained.
a) What is the main component of the colourless solution? b) Explain your answer in (a) above
answers
When magnesium metal is burnt in air, it reacts with both oxygen and nitrogen gases giving a white ash. Write two equations for the reactions that take place. (2mks)
Give one example of elements A and B
A B
ANSWER
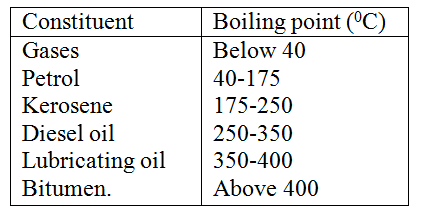
(a) The table below gives information about the major constituents of crude oil. Study it and answer the questions that follow. (i) Which one of the constituents or crude oil has molecules with the highest number of carbon atoms? (2mks) (ii) Name the process you would use to separate a mixture of petrol and diesel and explain how the separation takes place. (2mks) (iii) Explain why the constituent of crude oil and write its formula (1mk) (iv) Name one gas that is likely to be a constituent of crude oil and write its formula. (1mk) (b) What condition could cause a poisonous gas to be formed when Kerosene is burnt? Explain (2mks) (c) Give one use of bitumen (1mk) KCSE PAST PAPERS - CHEMISTRY FORM 1 TOPICAL QUESTIONS AND ANSWERSQUESTIONS
|
Chemistry Topics
All
Archives
December 2024
|
||||||||||||||||||||||||||||||||
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
|
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |

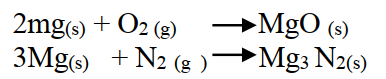




 RSS Feed
RSS Feed

