|
These are chemistry questions and answers categorized according to topics, papers i.e. Paper 1 and 2, Levels i.e. form 1 to form 4, kcse year the examination was done and section A or B
Select topic/category to open topical questions from that particular option provided. Chemistry Topics
0 Comments
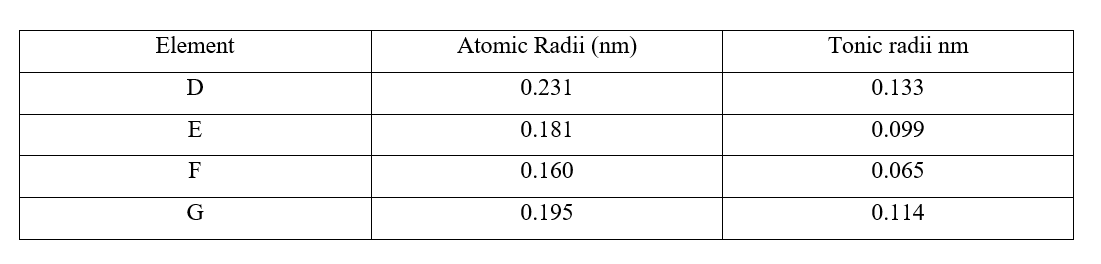
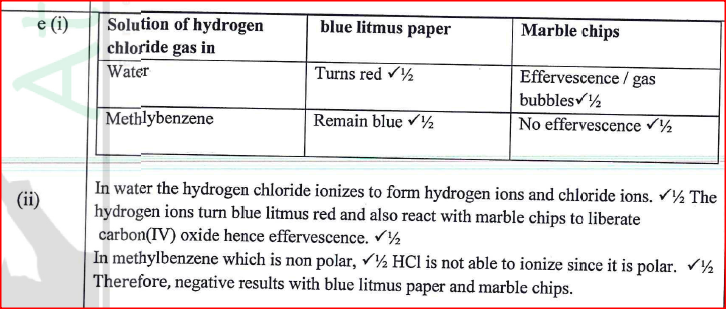
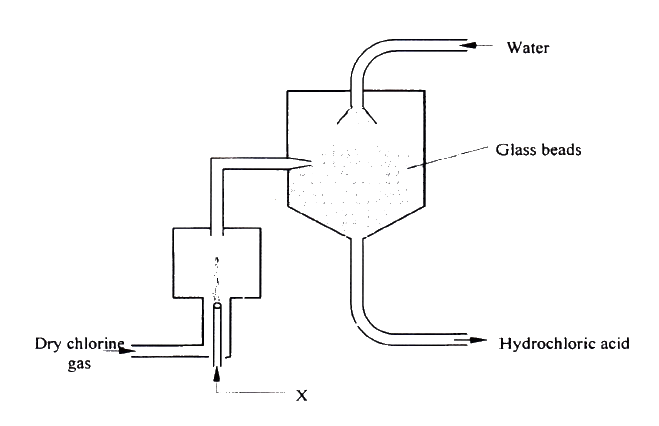
a) i) Are the members in this group likely to be conductor or non – conductors? (1mk)
ii) Which element would have the lowest atomic number? Explain. (1mk)
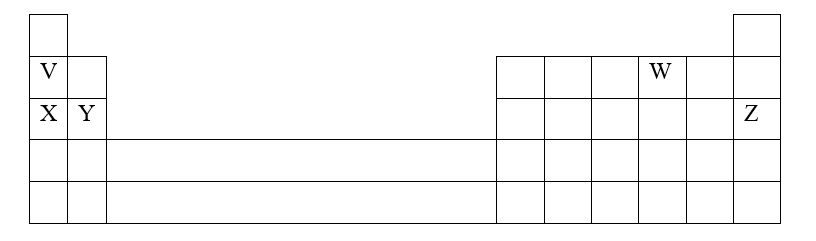
b) The grid below represents part of the periodic table. Study it and answer the questions that follow. (The letters are not the actual symbols of the elements)i) Select the element in period three which has the shortest atomic radius. Give a reason for your answer. (2mks)
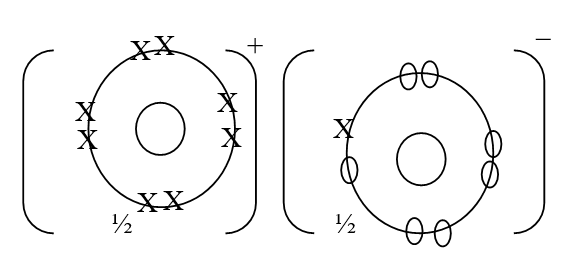
ii) Using dots (•) and crosses (x) to represent outermost electrons, draw a diagram to show the bonding in the compound formed when chlorine reacts with element X (1mk)
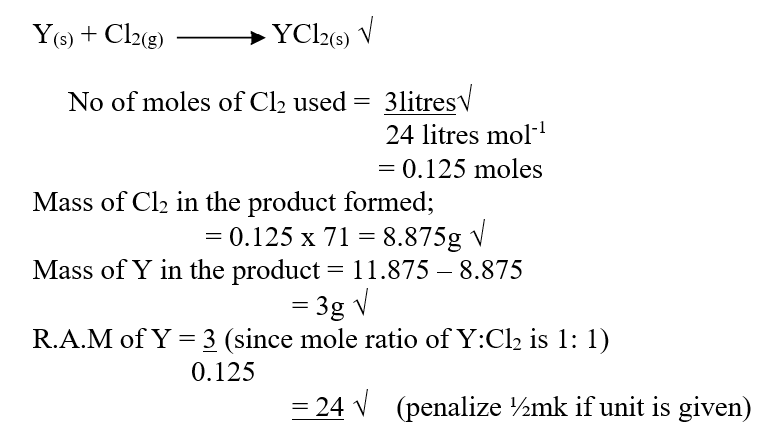
iii) When three liters of chlorine gas were completely reacted with element Y, 11.85g of the product were formed. Calculate the relative atomic mass of element Y. (3mks)(R.A.M of chlorine = 35.5, molar gas volume = 24 liters)
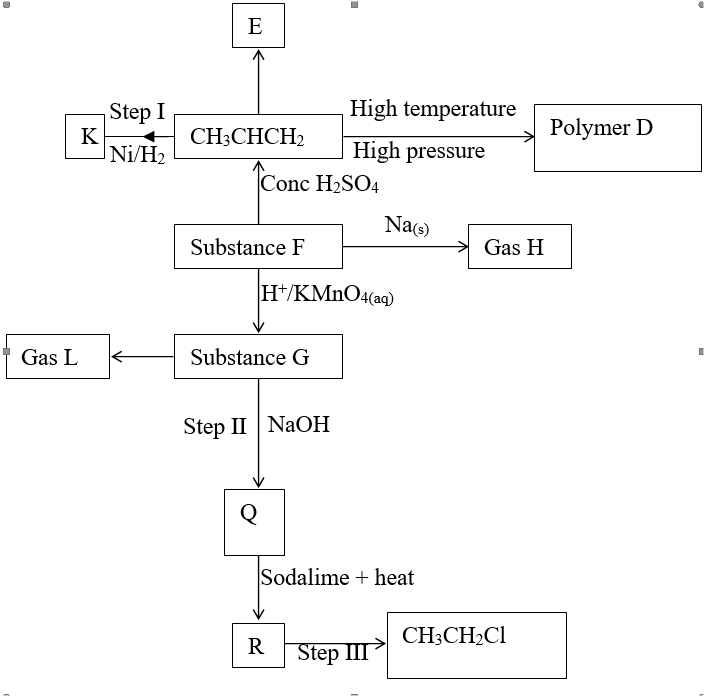
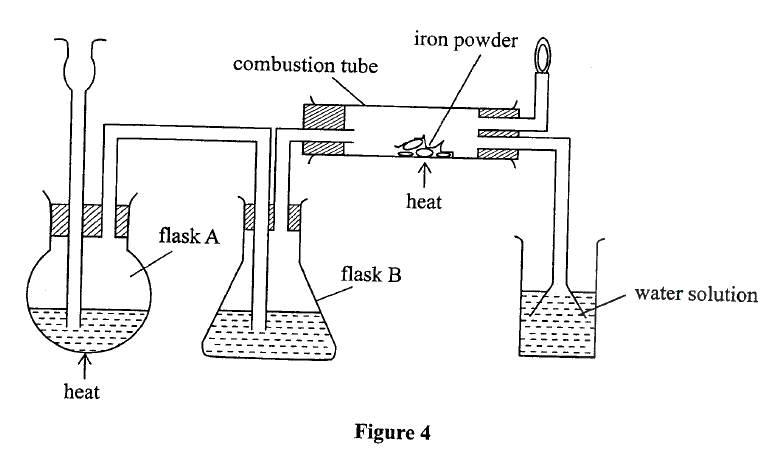
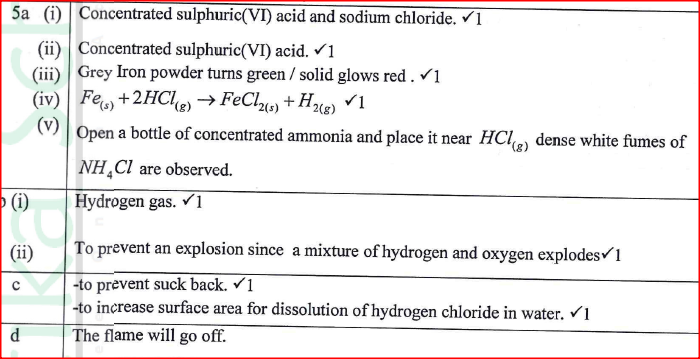
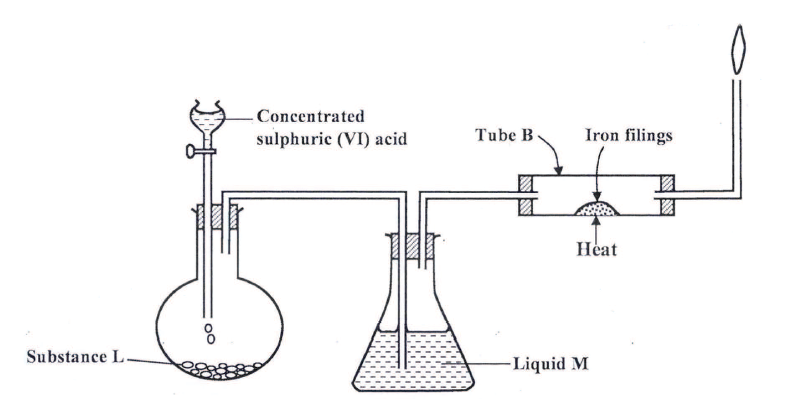
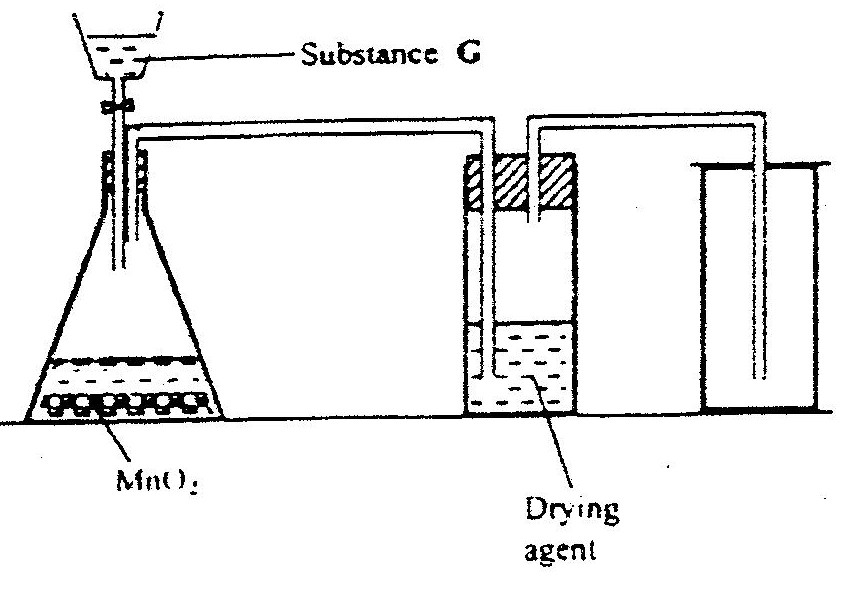
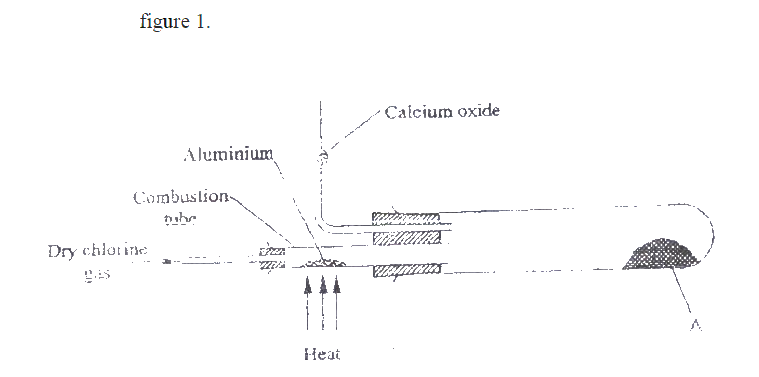
a) Name the following;i) Gas L (1mk)
Gas L – Carbon (IV) Oxide
ii) Gas H (1mk)
Gas H – Hydrogen
iii) K (1mk)
K – Propane
b) Name the processes involved in the following stepsi) Step I (1mk)
Step I : Hydrogen
ii) Step II (1mk)
Step II – Neutralization
iii) Step III (1mk)
Step III – Substitution
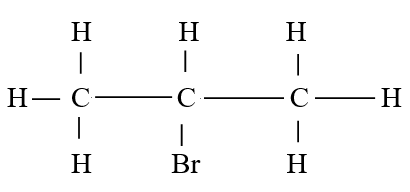
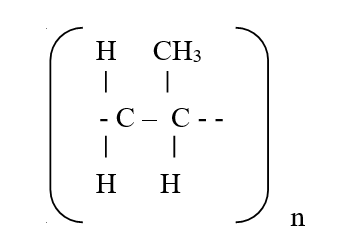
c) Draw the structure of compound E (1mk)d) Write a chemical equation for the complete combustion of substance F (1mk)e) Name the condition and reagents in step IIIi) Condition (1mk)
U.V light
ii) Reagent (1mk)
Chlorine
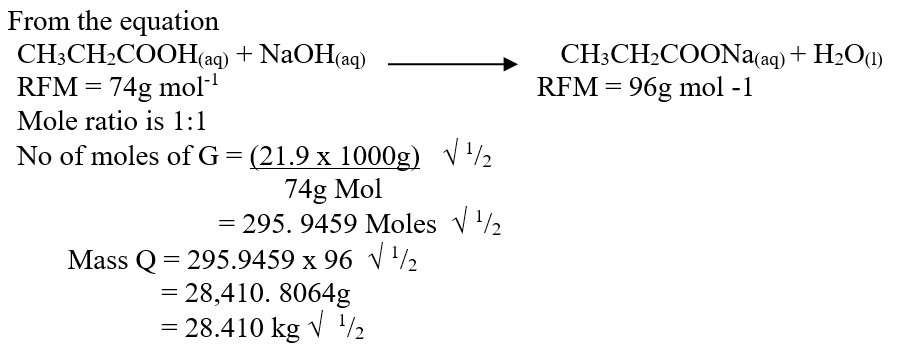
f) Calculate the mass of salt Q that would be formed by using 21.9kg of G when it reacts with excess sodium hydroxide (2mks)
|
Chemistry Topics
All
Archives
December 2024
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
|
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |
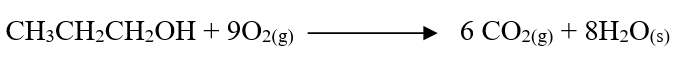






















 RSS Feed
RSS Feed

