|
These are chemistry questions and answers categorized according to topics, papers i.e. Paper 1 and 2, Levels i.e. form 1 to form 4, kcse year the examination was done and section A or B
Select topic/category to open topical questions from that particular option provided. Chemistry Topics
0 Comments
The empirical formula of a hydrocarbon is (CH2). It has a density of 0.001167g/cm3 at room temperature and pressure. (Molar gas volume at r.t.p is 24dm3)a) Determine the molecular formula of the hydrocarbon (3mks)
b) Draw the structural formula of the hydrocarbon (1mk)c) Ethene gas burns in Oxygen to form Carbon (IV) oxide and water.i) Write an equation for the reaction between ethane gas oxygen gas (1mk)ii) 15cm3 of ethene gas were mixed with 50cm3 of oxygen gas and the mixture was ignited into complete combustion. Calculate the volume of excess unreacted gas (3mks)d) What happens when ethene gas is bubbled through bromine water? (2mks)
e) Give any two uses of ethene gas (2mks)
Uses of Ethene gas
Name one gas used together with oxygen in welding other than acetylene gas (1mk)
Hydrogen
State two other uses of the gas named above (2mks)
(a) State Graham’s law of diffusion
(b) Explain why a balloon filled with helium gas deflates faster than a balloon of the same size filled with argon gas.
ANSWERS
(a) Graham’s Law of diffusion
The rate of diffusion of a gas is inversely proportional to the square root of its density at constant temperature and pressure. (b)Helium is less dense than argon hence it diffuses out of the balloon faster than argon.
(a) State Charles' Law.
(b) Explain why the pressure of a fixed mass of a gas increases, when the volume of the gas is reduced at constant temperature.
ANSWERS
(a)The volume of a fixed mass of a gas is directly proportional to the absolute temperature at constant pressure.
(b)As the volume decreases, there is increased bombardment / collisions of the molecules against the walls of the container, hence increased pressure.
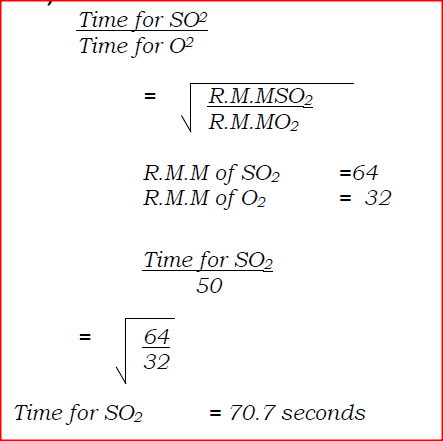
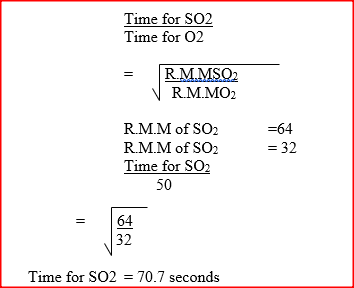
60 cm3 of oxygen gas diffused through a porous partition in 50 seconds. How long would it take 60cm3 of sulphur (IV) oxide gas to diffuse through the same partition under the same conditions? (S = 32.0, 0 = 16.0)
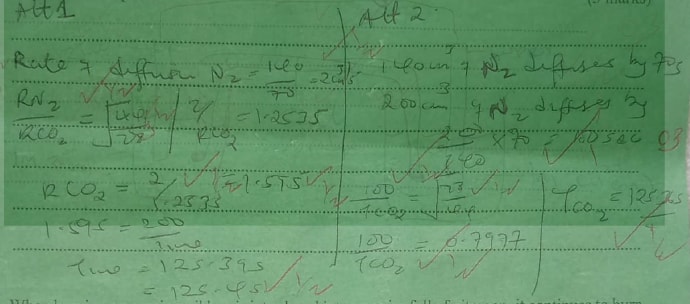
140cm3 of nitrogen gas diffuses through a membrane in 70 seconds. Flow long will it take 200cm3 of carbon(IV) oxide gas to diffuse through the same membrane under the same conditions of temperature and pressure. (3 marks)
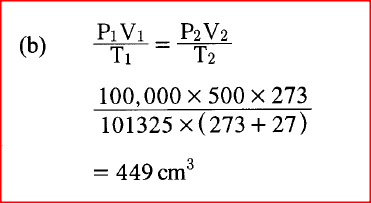
a) State the Boyles Law
b) A gas occupies 500cm3 at 27°C and 100,000 Pa, What will be its volume at O°C and 101325 Pa?
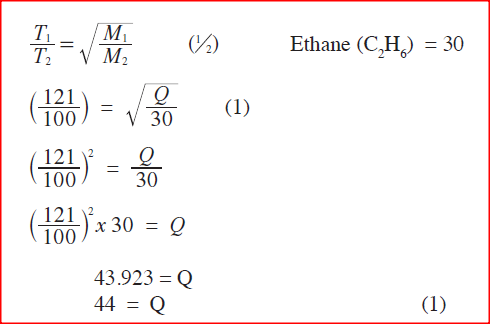
100cm3 of a sample of ethane gas diffuses through a porous pot in 100 seconds. What is the molecular mass of gas Q if 1000 cm3 of the gas diffuses through the same porous not in 121 seconds under the same conditions? (C=12.0, H=1.0)
(a) State the Charles’ law
(b) A certain mass of gas occupies 146 dm3 at 291 K and 98.31 kPa. What will be its temperature if its volume is reduced to 133 dm3 at 101.325 kPa?
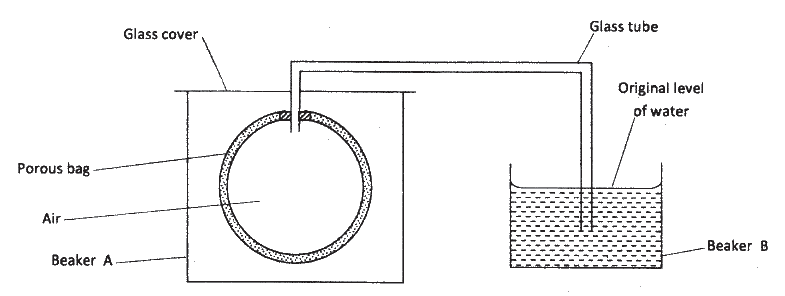
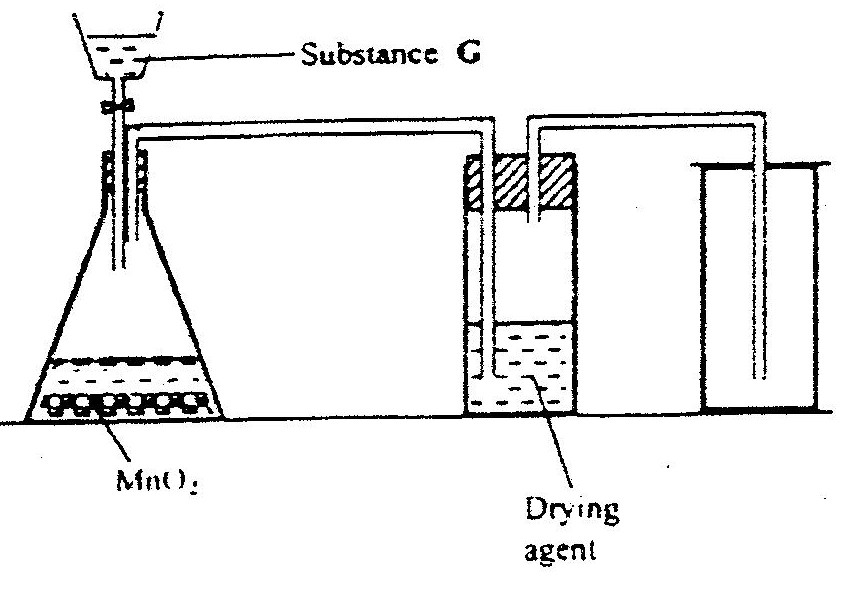
The set up shown below was used to investigate a property of hydrogen gas.
State and explain the observation that would be made in the glass tube if beaker A was filled with hydrogen gas.
ANSWERS
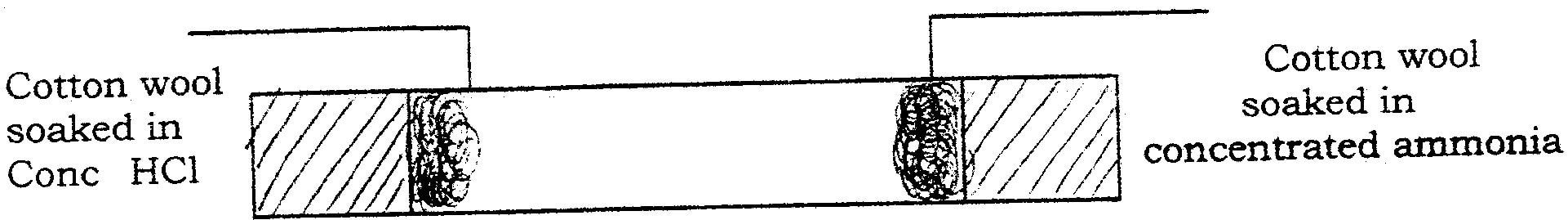
In an experiment, ammonia chloride was heated in a test tube. A moist red litmus paper placed at the mouth of the test tube first changed blue then red. Explain these observations
Expected Response
NH4Cl decomposes to form NH3(g) and HCl(g).Ammonia diffuses faster than HCl because its light. Ammonia is basic and thus red litmus paper turns blue while HCl is acid thus blue litmus turns red.
A given volume of ozone, (O3) diffused from a certain apparatus in 96 seconds. Calculate the time taken by an equal volume of carbon dioxide (CO2) to diffuse under the same conditions (O = 16.0, C = 12.0)
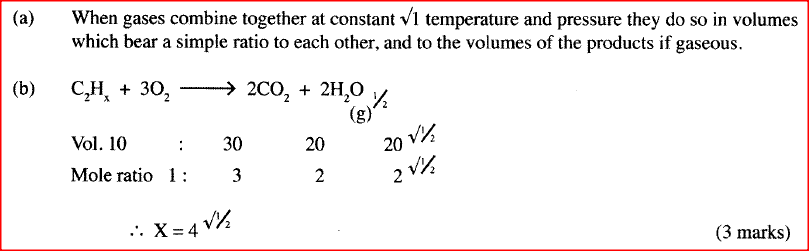
(a) State the Gay Lussac's Law.
(b) 10cm3 of a gaseous hydrocarbon, C2HX required 30cm3 of oxygen for complete combustion. If steam and 20cm3 of carbon (IV) oxide were produced, what is the value of X?
A certain mass of gas occupies 0.15dm3 at 293K and 98,648.5Pa. Calculate its Volume at 101,325Pa and 273K.
The set up below was used to investigate some properties of two gases M and N
When beaker A was filed with gas M, the level of water in the glass tube rose to
point II. When the experiment was repeated using gas N, the level of water dropped to point III. Explain these observations.
The pressure of nitrogen gas contained in a 1dm3 cylinder at -196°C was 107 Pascals.
Calculate the: a) Volume of the gas at 25°C and 105 Pascals. b) Mass of nitrogen gas(Molar volume of gas is 24dm3, N = 14.0)
ANSWERS
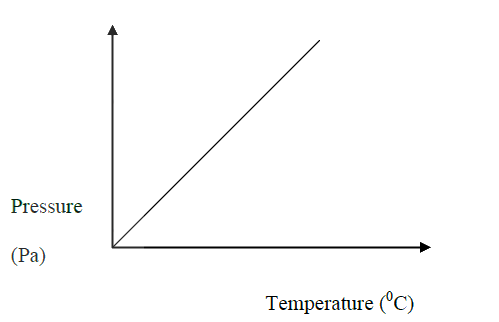
(a) Temperature and pressure are directly proportional (l) IR words towards that of real
(b) With increase in temperature, the gas particles gain more Kinetic energy They move faster and collide with the walls of the container more frequently hence increasing pressure.
A sealed glass tube containing air at s.t.p was immersed in water at 1000c. Assuming that there was no increase in the volume of the glass tube due to the expansion of the glass, calculate the pressure of the inside tube.
(standard pressure = 760mmHg,)
Expected Response
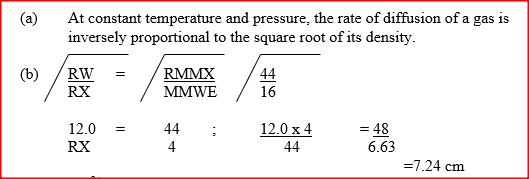
a) State the Graham's law diffusion.
b) The molar masses of gases W and X are 16.0 and 44.0 respectively. If the rate of diffusion of W through a porous material is 12cm3s-1 calculate the rate of diffusion of X through the same material. Related Chemistry Questions and Answers on Gas Laws Form 3 Level
A small crystal of potassium manganate (VII) was placed in a beaker water. The beaker was left standing for two days without shaking. State and explain the observations that were made.
ANSWERS
a) State the Charles law
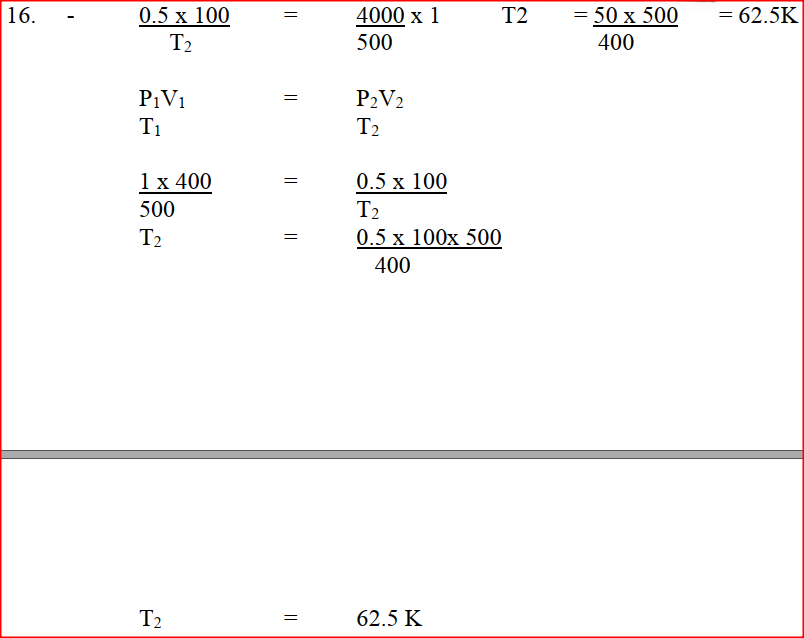
b) The volume of a sample of nitrogen gas at a temperature of 291 K and 1.0x105 Pascal‟s was 3.5 x 10-2m3. Calculate the temperature at which the volume of the gas would be 2.8 x 10-2m3 at 1.0 x 105 Pascal. A gas occupies a volume of 400cm3 at 500k and 1 atmosphere pressure. What will be the temperature of the gas when the volume and pressure of the gas is 100cm3 and 0.5 atmospheres respectively.
60cm3 of oxygen gas diffused through a porous partition in 50 seconds. How long would it take 60cm3 of sulphur (IV) oxide gas to diffuse through the same partition under the same conditions? (S= 32.0, 0 = 16.0)
|
Chemistry Topics
All
Archives
December 2024
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
|
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |
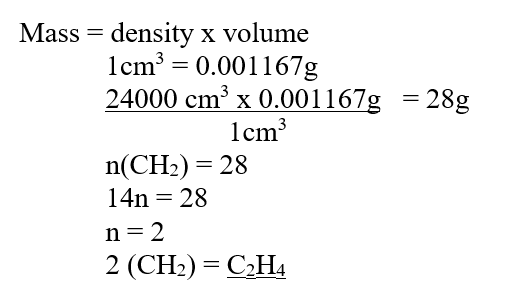
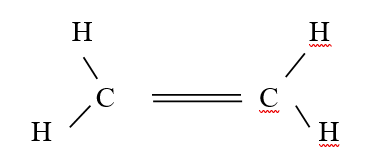
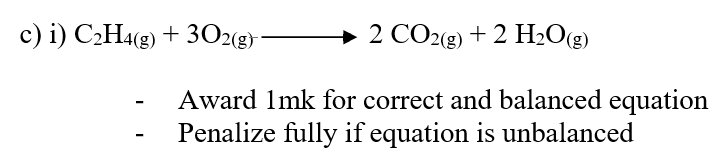
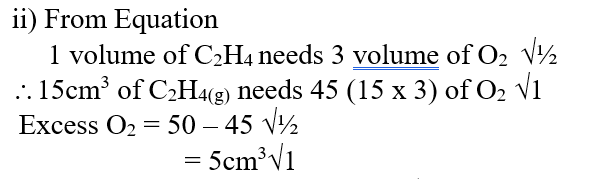

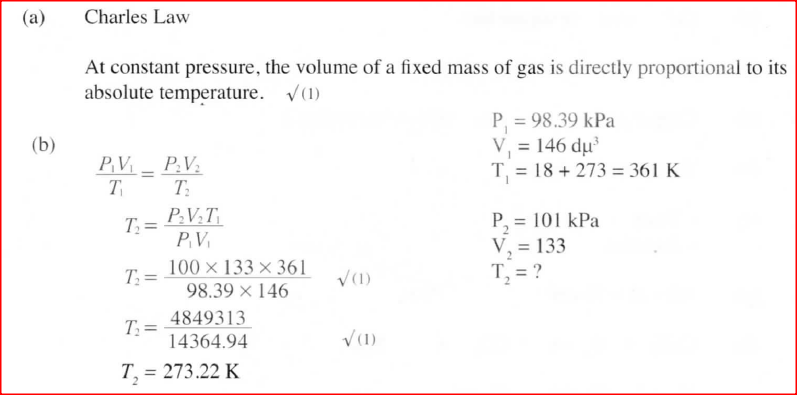
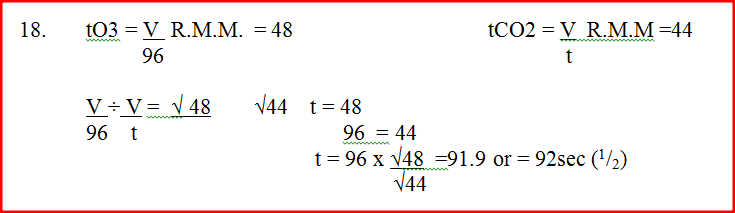
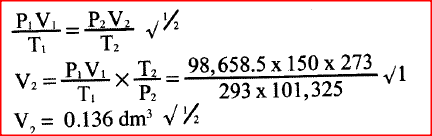
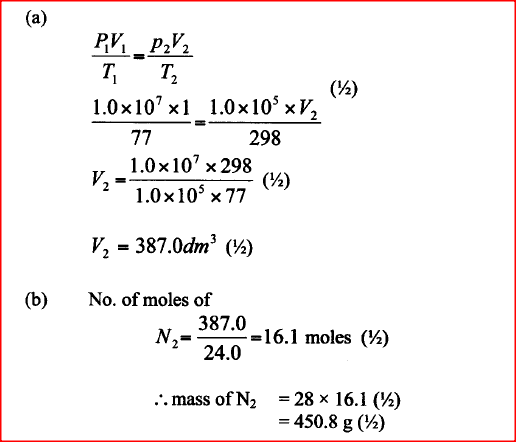
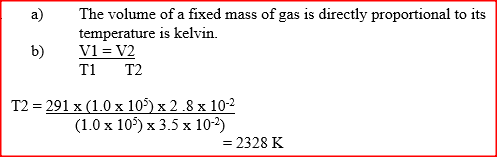


 RSS Feed
RSS Feed

