|
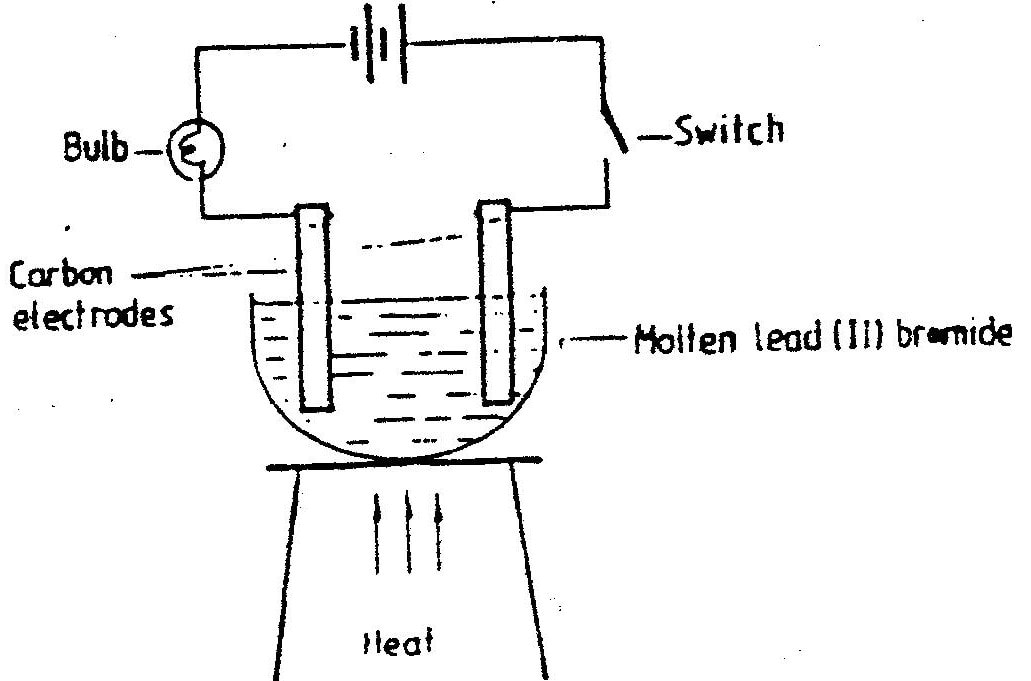
Study the set – up below and answer the question that follows.
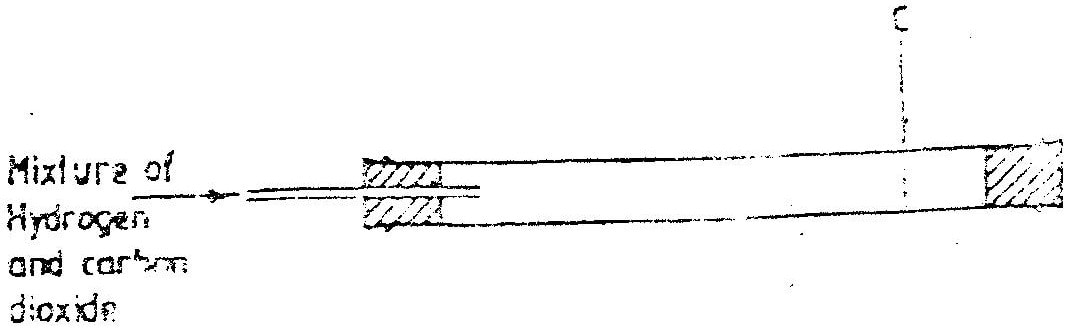
State and explain the observations that would be made when the circuit is completed.
0 Comments
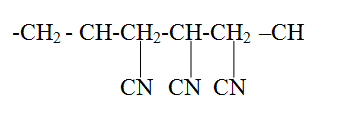
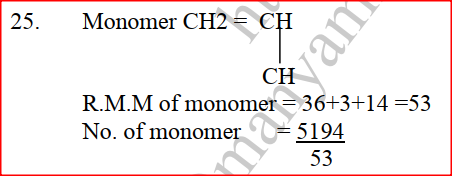
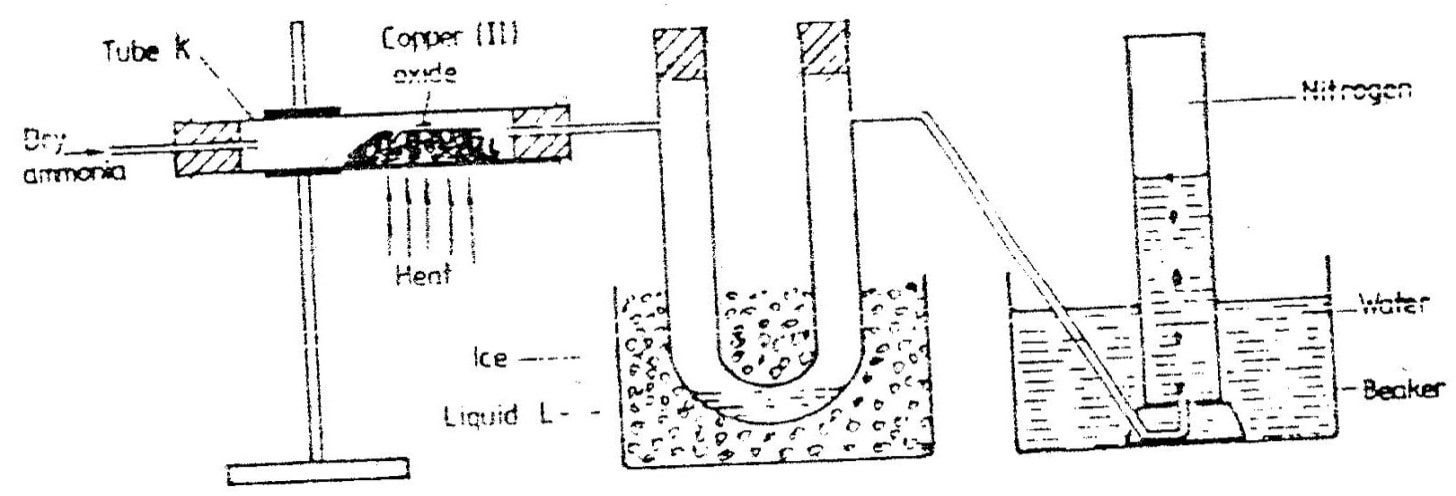
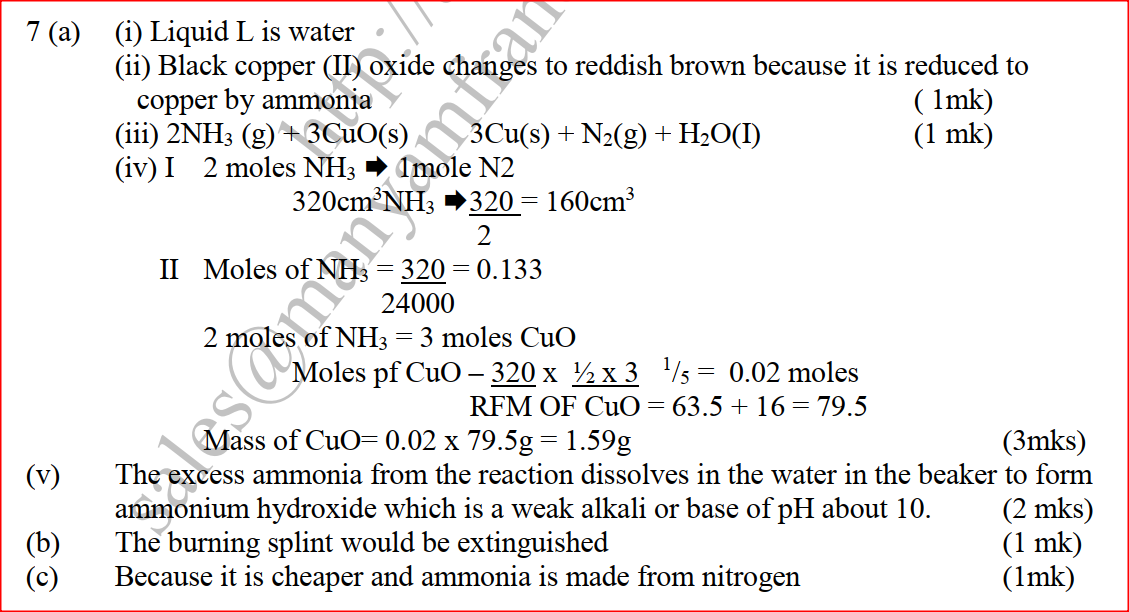
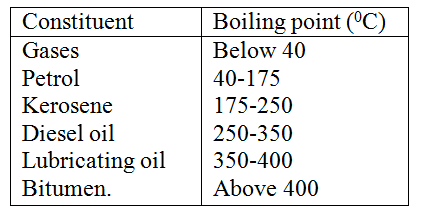
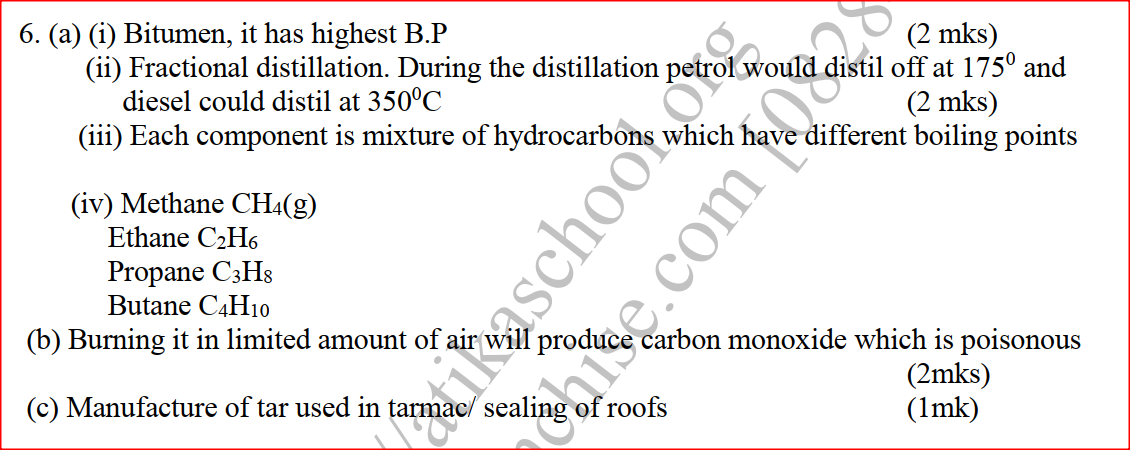
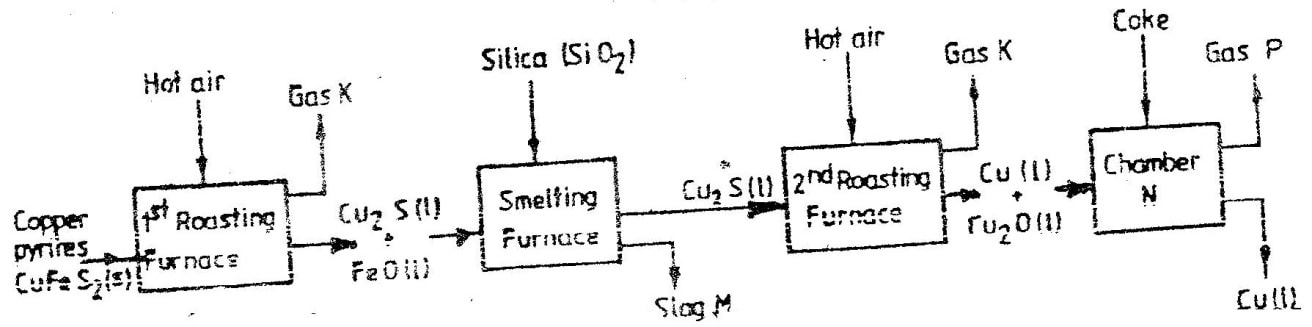
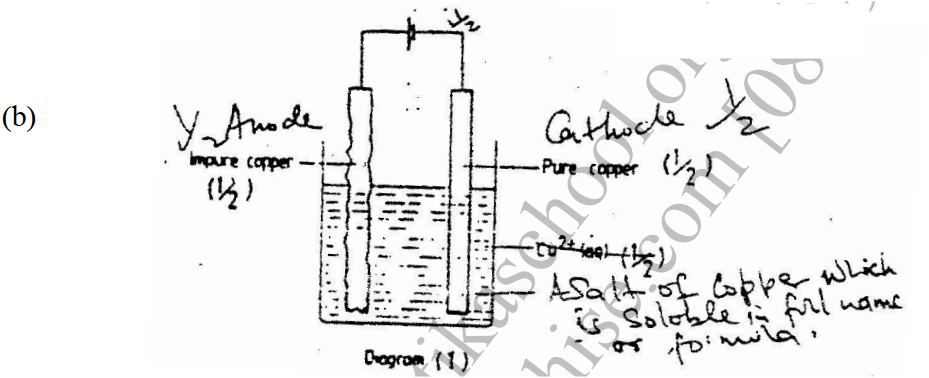
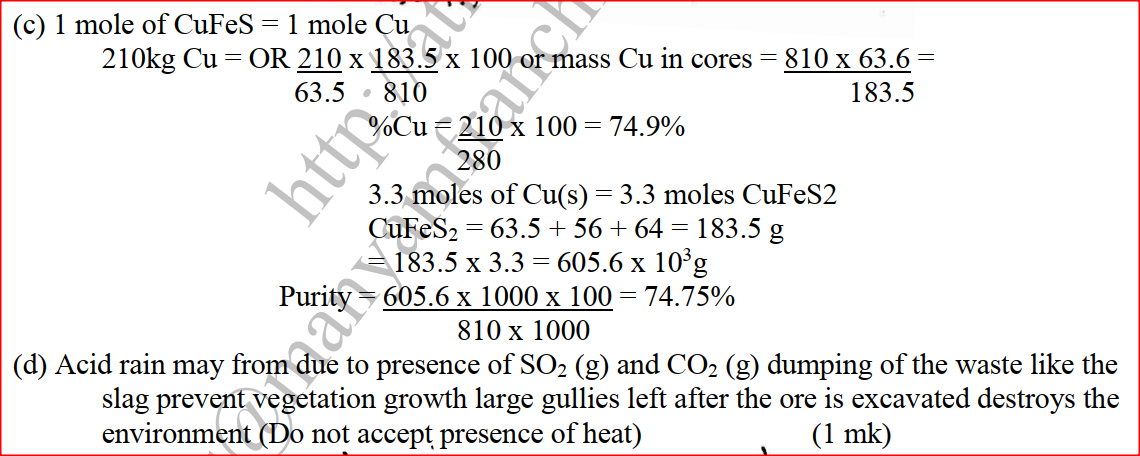
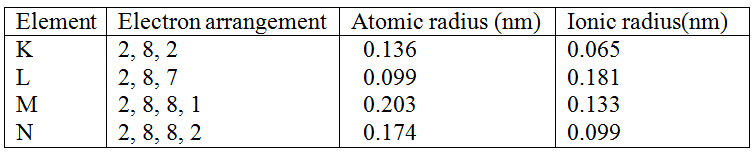
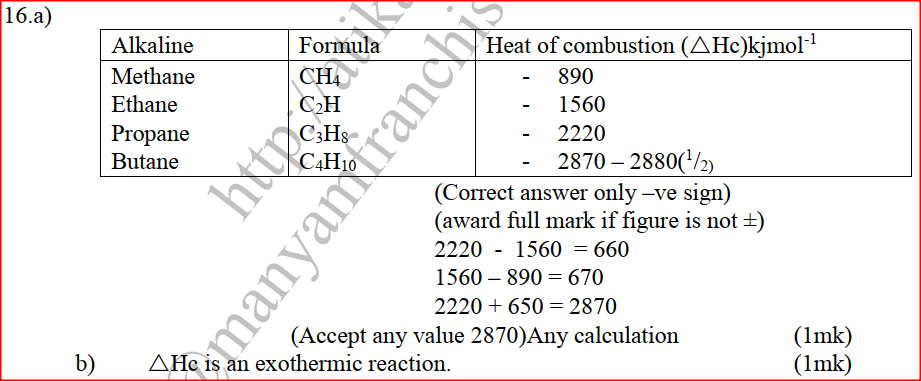
(a) The diagram below shows a set-up that can be used to obtain nitrogen gas in an experiment. (i) Name liquid L (1mk) (ii) What observation would be made in tube K after heating for some time? (1mk) (iii) Write an equation for the reaction that took place in tube K. (1 mk) (iv) If 320 cm3 of ammonia gas reacted completely with the copper? Calculate: I Volume of nitrogen gas produced. (1mk) II the mass of copper oxide that reacted (3mks) (Cu = 63.5, O=16.O, one mole of gas occupies 24 liters at room temperature and pressure) (v) At the end of experiment the PH of the water in the beaker was found to be about ______________________ Explain (2mks) (b) In another experiment a gas jar containing ammonia was inverted over a burning splint. What observation would be made? (1mk) (c) Why is it advisable to obtain nitrogen from air instead ammonia? (1mk) The flow charts below show an analysis of a mixture R that contains two salts. Study the analysis and answer the questions that follow. (a) (i) What condition is necessary for the process in step I to take place?(1mk) (ii) Draw a labeled diagram for the set-up that could be used to separate the mixture formed in step II (2mks) (iii) Write ionic equation for the reaction between the cation in filtrate X and aqueous ammonia. (1mk) (iv) What observation would indicate the presence of NO2 (g) in step I (v) State how water vapour, in step I could be identified. (1mk) (b) (i) What conclusion can be drawn from step IV only? Explain? (2mks) (ii) Write the formula of an anion present in the residue U. Explain? (2mks) (iii) Suggest the identity of the cation present in solution z. (1mk) (c) Name the two salts present in the mixture R. (2mks) (a) The table below gives information about the major constituents of crude oil. Study it and answer the questions that follow. (i) Which one of the constituents or crude oil has molecules with the highest number of carbon atoms? (2mks) (ii) Name the process you would use to separate a mixture of petrol and diesel and explain how the separation takes place. (2mks) (iii) Explain why the constituent of crude oil and write its formula (1mk) (iv) Name one gas that is likely to be a constituent of crude oil and write its formula. (1mk) (b) What condition could cause a poisonous gas to be formed when Kerosene is burnt? Explain (2mks) (c) Give one use of bitumen (1mk) The flow chart below outlines some of the process involved during extraction of copper from copper pyrites. Study it and answer the questions that follow. (i) Name gas K (1mk) (b) The copper obtained from chamber N is not pure. Draw a labeled diagram to show the set up you would use to refine the copper by electrolysis. (3mks) (c) Given that the mass of copper obtained from above extraction was 210kg, determine the percentage purity of the ore(copper pyrites) if 810kg of it was fed to the 1st roasting furnace. (Cu = 63.5, Fe = 56.0, s=32.0) (3mks) (d) Give two effects that this process could have on the environment (2mks) In an experiment to determine the heat of combustion of methanol, CH2OH a student used a set up like the one shown in the diagram below. a) Write an equation for the combustion of methanol b) Calculate: (i) The number of moles of methanol used in this experiment (C = 12; O = 16; H = 1) (ii) The heat of combustion per mole of methanol. (1mk) (iii) The heat of combustion per mole of methanol (2mks) (c) Explain why the value of the molar heat of combustion for methanol obtained in this experiment is different from the theoretical value. (d) On the axis below draw an energy level diagram for the combustion of methanol. a) In an experiment hydrogen chloride gas was prepared and reacted with aluminium turnings to form a solid q and gas R as shown in the diagram (i) Name: Liquid P : Solid Q (1mk) : Gas R (1mk) (ii) Name another substance that could serve the same purpose as the concentrated sulphuric acid. (1mk) (iii) Explain the following observation. When blue litmus paper was dipped into the water in the beaker at the end of the experiment it turned red. Explain why solid Q collects farther away from the heated aluminium (2mks) (b) (i) Write an equation for the reaction that takes place between ammonia gas and hydrogen gas (1mk) (ii) Calculate the mass of the product that would be formed when 2000cm3 of hydrogen chloride gas reacts completely with excess ammonia gas (H=1, O; N= 14.0, C1 = 35.5, one mole of gas occupied 24 litres at room temperature and pressure.) (3mks) The table below gives information on four elements by letters K, L, M and N. Study it and answer the questions that follow. The letters do not represent the actual symbols of the elements. a) Which two elements have two similar properties? Explain (2mks) A compound C4H10O_ is oxidized by excess acidified potassium permanganate to form another compound C4H8O2. The same compound C4H10O reacts with potassium to produce hydrogen gas. a) Draw the structural formula and name the compound CaH10O (1mk) b) Write an equation for the reaction between potassium and compound C4H10O. a) 100gm of radioactive 233 Pa was reduced to 12.5g after 81 days. 91 Determine the half-life of Pa. (2mks) b) 233 Pa decays by beta emission. What is the mass number and 91 Atomic number of the element formed? (1mk) Describe how the following reagents can be used to prepare lead sulphate solid potassium sulphate, solid lead carbonate, dilute nitric acid and distilled water.
Expected Response
Dissolve the potassium sulphate (1/2) in water, dissolve (1/2) the lead carbonate in the nitric acid, mix the two solutions (1/2) and filter (1/2) off the lead sulphate precipitate//Dissolve lead carbonate in nitric acid add solid pbSO4 and filter off (max1 ½)//Dissolve this in HNO3 and add solid pbCO3 and filter off the precipitate.
Give a reason why calcium hydroxide solution is used to detect the presence of carbon dioxide gas while sodium hydroxide in NOT?
Expected Response
Ca (OH)2(aq) forms white precipitate (1/2) with CO2 Can be observed NaOH(1/2)(aq) does not form a precipitate. (1mk) Explain the following observation. A chloride dissolves in water to form an electrolyte while the same chloride dissolves in methylbenzene to for a non – electrolyte. (1mk)
Expected Response
The chloride form ions in water which conduct electric current. NO ions are formed in methylbenzene /chloride exists in methylbenzene as molecules. (2mks)
Explain why the enthalpy of neutralization of ethanoic acid with sodium hydroxide is different from that of hydrochloric acid with sodium hydroxide. (2mks)
Expected Response
Enthalpy of neutralization between CH3CaOH(aq) and NaOH(aq) is lower than thatbetween HCl(aq) and NaOH because CH3CaOH(aq) is a weak acid which does not dissociate fully in water thus some of heat produced is used for dissociation fully dissociated and partially dissociated. (2mks)
State what would be observed when dilute hydrochloric acid is added to the products formed when a mixture of iron fillings and sulphur? (1mk)
Expected Response
A gas with a smell of rotten eggs is formed H2S gas is formed / A greenish solution is formed? Effervescence / A gas is produced / Black solid dissolves. (1mk)
Expected Response
W because its solubility decreases with increase in temperature
Expected Response
Hydrogen, because it is lighter/ less denser / diffuses faster (2mks)
Expected Response
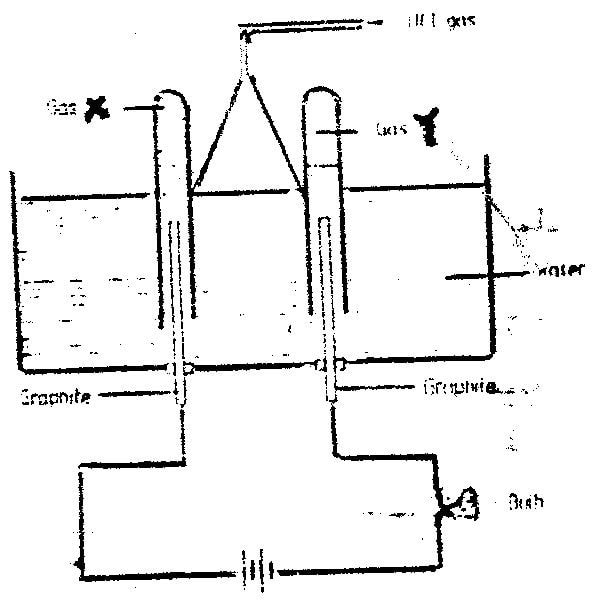
a) I – Molten sulphur
II – Super heated water / water
b) To force the molten sulphur out
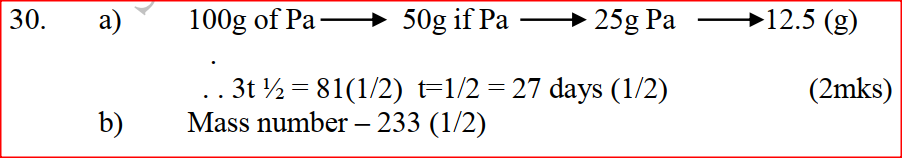
Explain how you would separate mixture of nitrogen and oxygen gases given that their boiling points are –1960C and 1830C respectively (2mks)
Answer
Cool the mixture to a temperature below – 1960C to form a liquid then start warming, Nitrogen distils off a gas at – 1960 (cool first)
|
Chemistry Topics
All
Archives
December 2024
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
|
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |






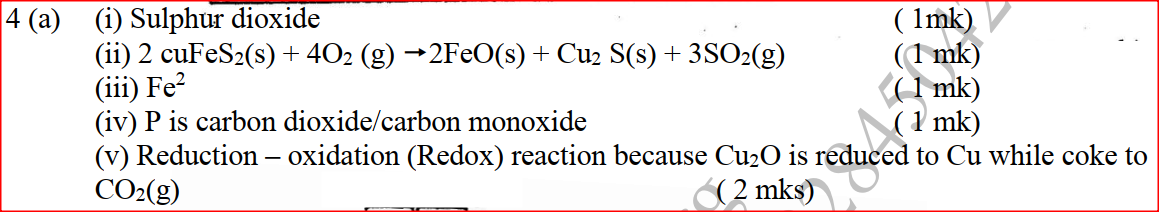


















 RSS Feed
RSS Feed

