|
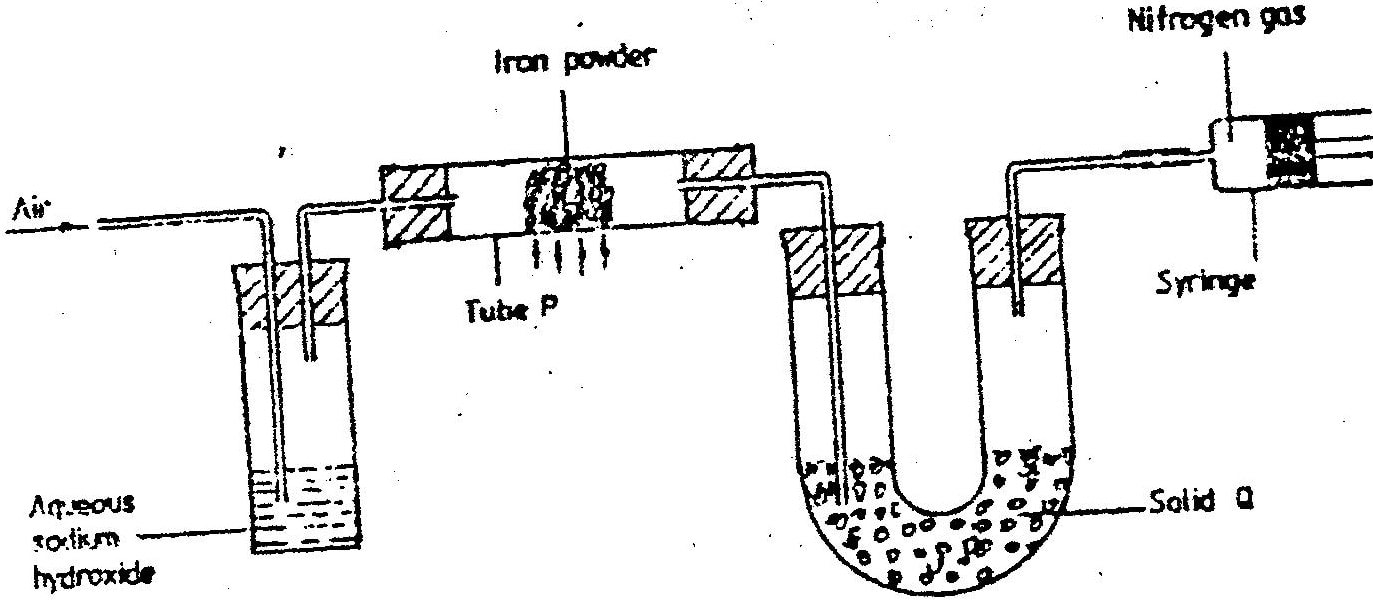
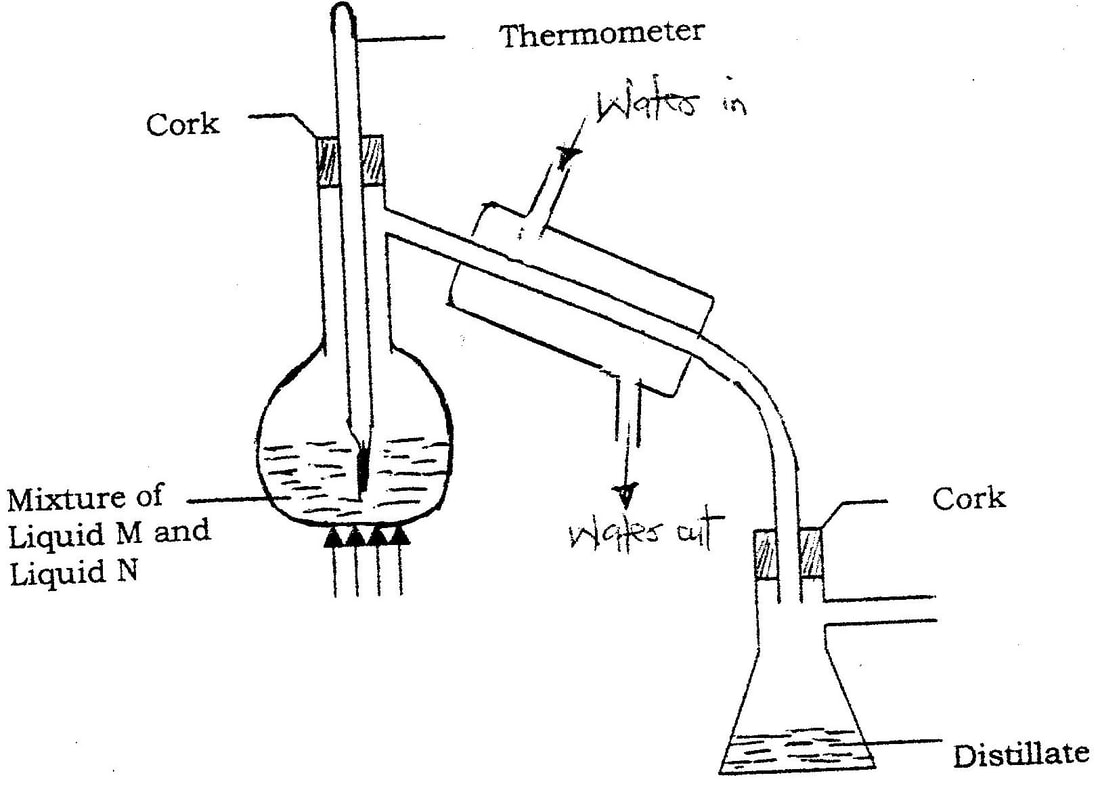
(a) The diagram below represents a set up that was used to obtain dry nitrogen from air. Study it and answer the questions that follow
Related Chemistry Questions and Answers on nitrogen and its compounds form 3 level
0 Comments
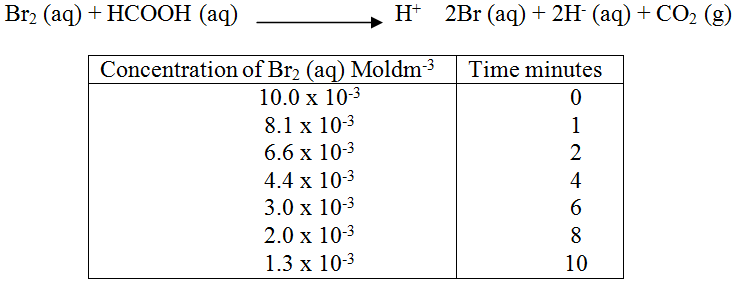
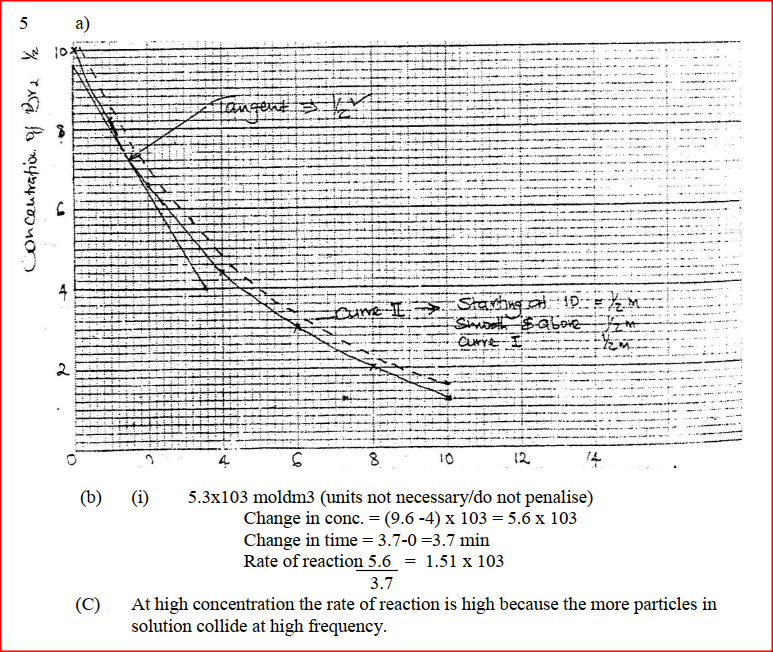
The reaction between and methanoic acid at 300C proceeds according to the information given below (a) On the grid below, plot a graph of concentration of Bromine (Vertical axis against time) (b) From the graph determine: (i) The concentration of bromine at the end of 3 minutes (ii) The rate of reaction at time ‘t’ where t = 1 ½ minutes (c) Explain how the concentration of bromine affects the rate of reaction (d) On the same axis sketch the curve that would be obtained if the reaction was carried out at 200C and label the curve as curve II. Give a reason for your answer.
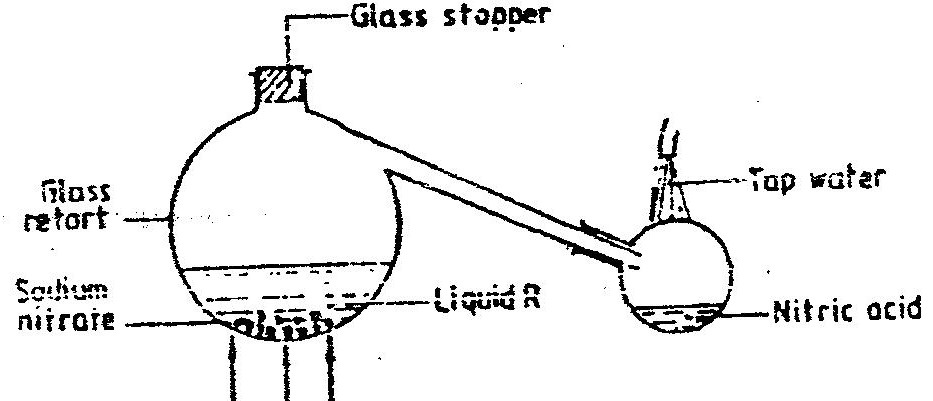
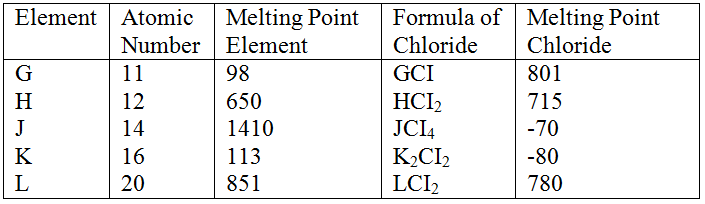
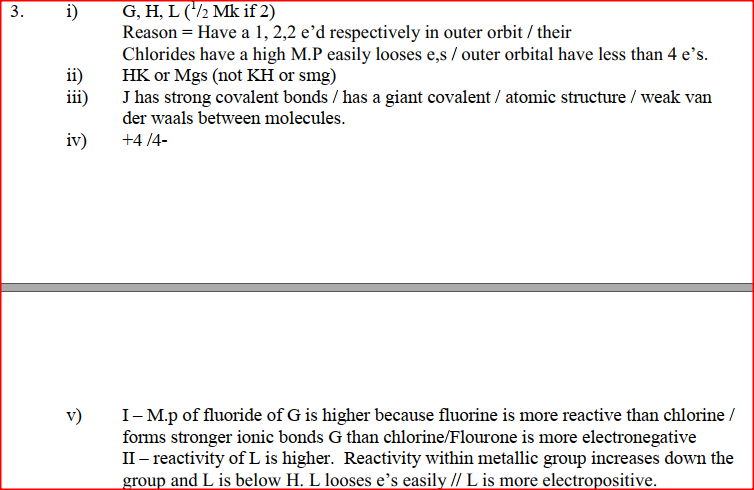
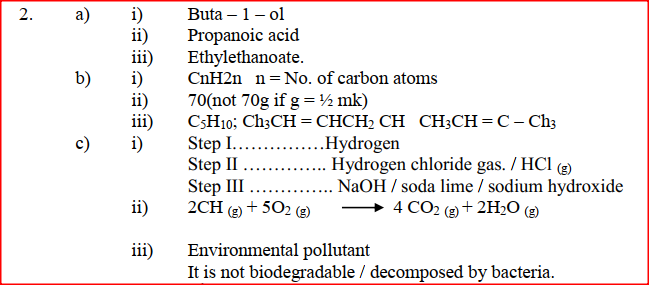
(a) Study the information below and answer the questions that follow:
The letters do not represent the actual symbols of the elements
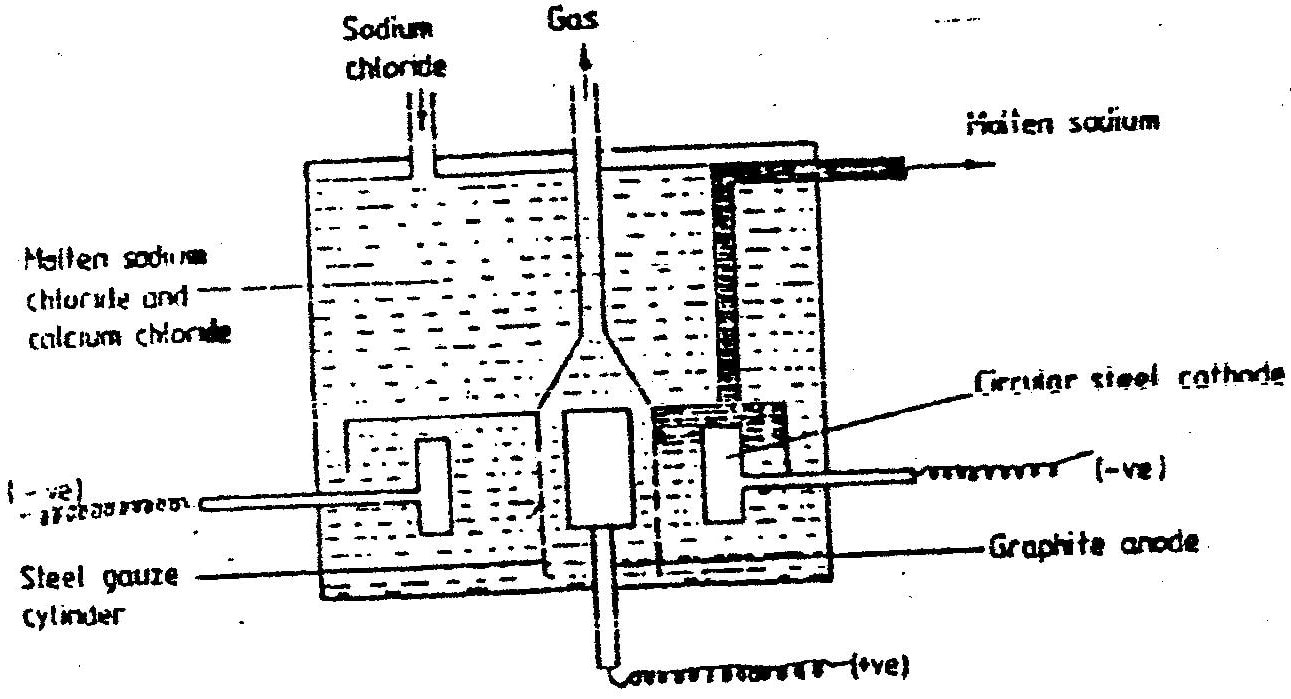
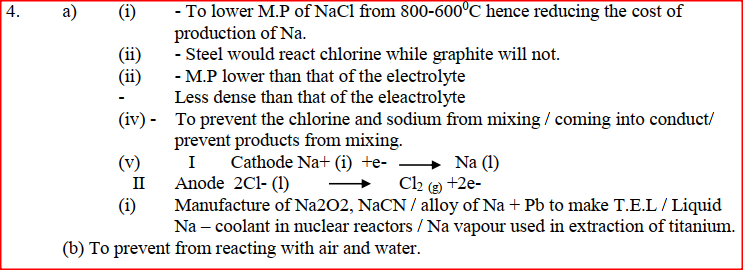
(a) The diagram below shows the extraction of sodium metal using the downs cell. Study it answer the questions that follow
(a) Give the names of the following compounds
(b) Study the information in the table below and answer the questions that follow
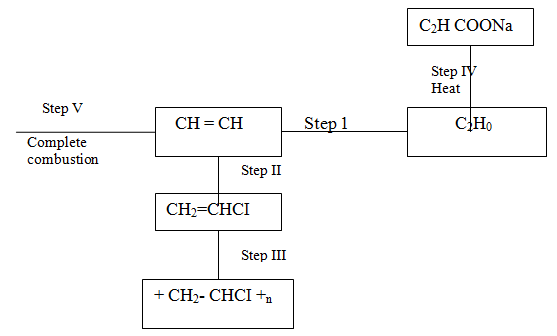
(i) Name the reagents used in:
Step I ……………………. Step II …………………….. Step III …………………….. (ii) Write an equation for the complete combustion of CH = CH (iii) Explain one disadvantage of the continued use of items made from the compound formed in step III
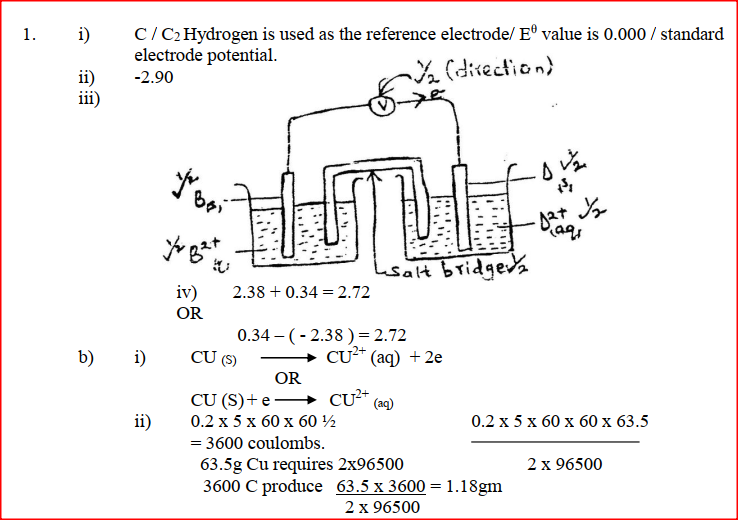
(i) Which element is likely to be hydrogen? Give a reason for your answer (ii) What is the Eθ value of the strongest reducing agent? (iii) In the space provided draw a labeled diagram of the electrochemical cell that would be obtained when half – cells of elements B and D are combined (iv) Calculate the Eθ value of the electrochemical cell constructed in (iii) above (b) During the electrolysis of aqueous copper (II) sulphate using copper electrodes, a current of 0.2 amperes was passed through the cell for 5 hours (i) Write an ionic equation for the reaction that took place at the anode (ii) Determine the change in mass of the anode which occurred as a result of the electrolysis process (Cu= 63.5, 1 Faraday = 96,500 coulombs). Aqueous potassium sulphate was electrolysed using platinum electrodes in a cell. a) Name the products formed at the cathode and anode. Anode b) How does the concentration of the electrolyte change during electrolysis. c) Why would it not be advisable to electolyse aqueous potassium sulphate using potassium metal electrodes.
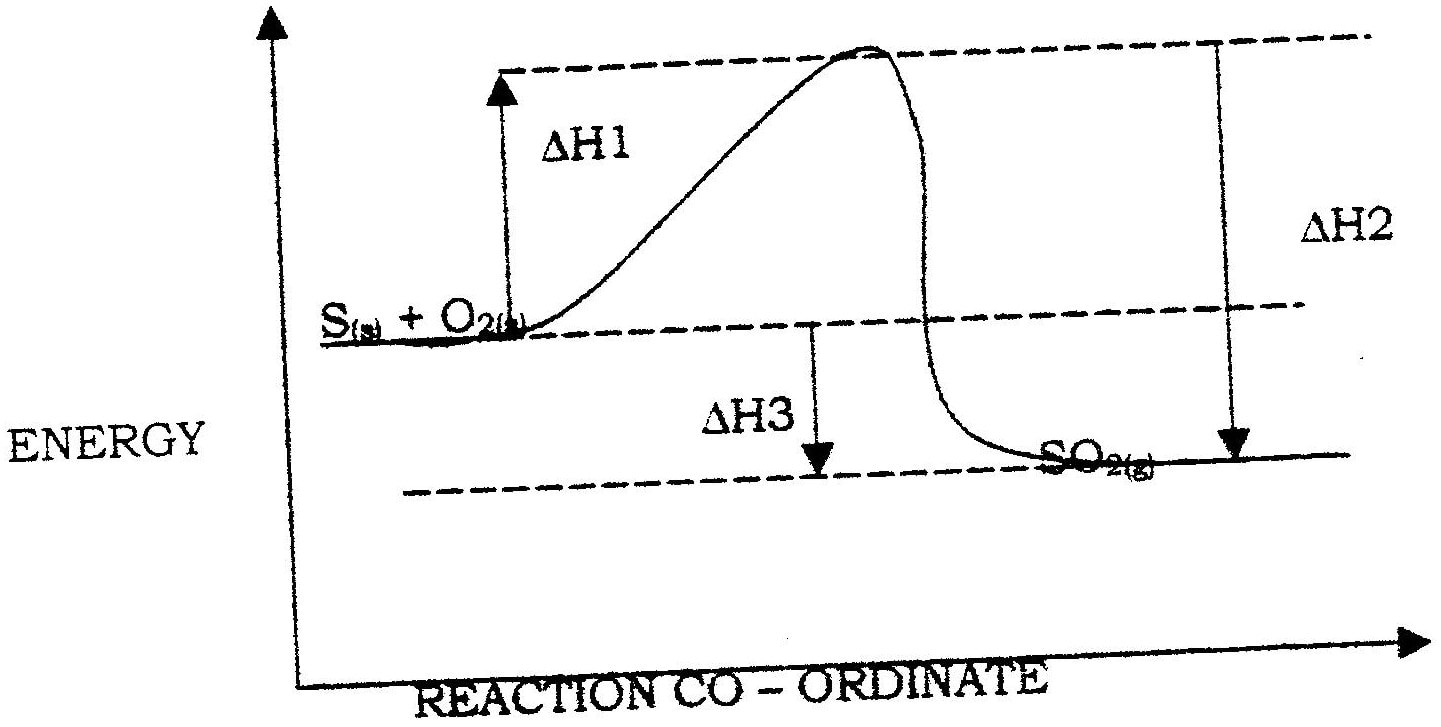
An element Y has the electronic configuration 2.8.5
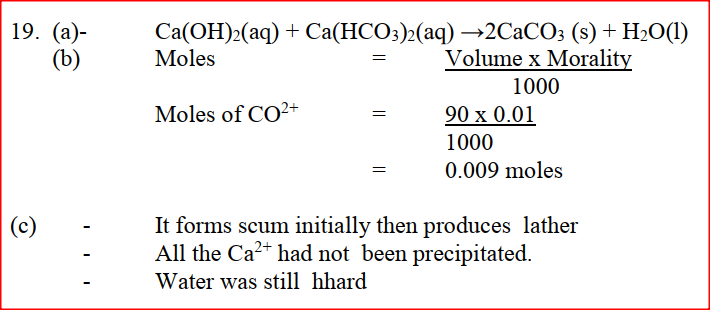
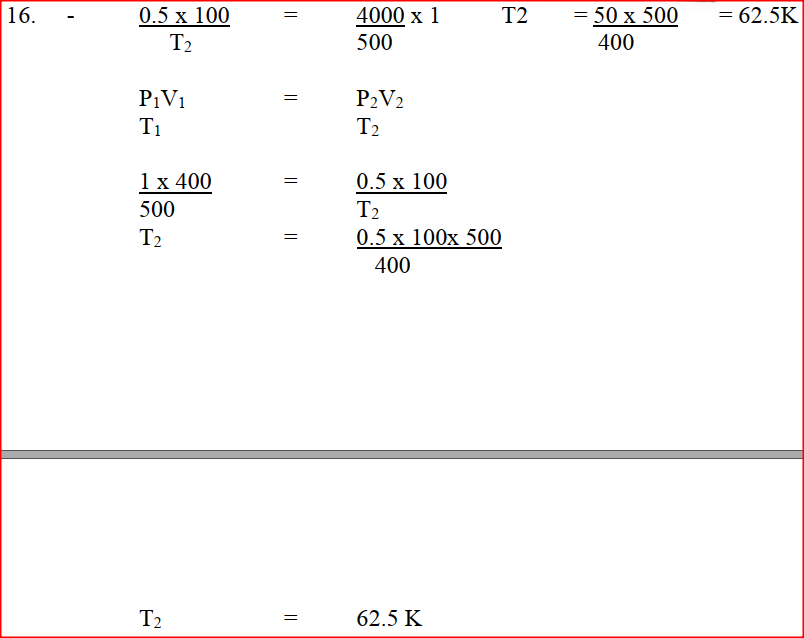
90cm3 of 0.01M calcium hydroxide were added to a sample of water containing 0.001 moles of calcium hydrogen carbonate. a) Write an equation for the reaction which took place b) Calculate the number of moles of calcium ions in 90cm3 of 0.01M calcium hydroxide. c) What would be observed if soap solution was added drop wise to a sample of the water after the addition of calcium hydroxide? Give a reason. A gas occupies a volume of 400cm3 at 500k and 1 atmosphere pressure. What will be the temperature of the gas when the volume and pressure of the gas is 100cm3 and 0.5 atmospheres respectively. In an equation below, identify the reagent that acts as abase. Give a reason. H2O(aq) + H2O(l) → H3O(aq) + HO2-(aq) When 0.6g of element J were completely burnt in oxygen and all the heat evolved was used to heat 500cm3 of water, the temperature of the water rose from 230C to 320C. Calculate the relative atomic mass of element J given that the specific heat capacity of water = 4.2JK-1g-1, density of water = 1.0g/cm3 and molar heat is combustion of J is 380Kjmol-1
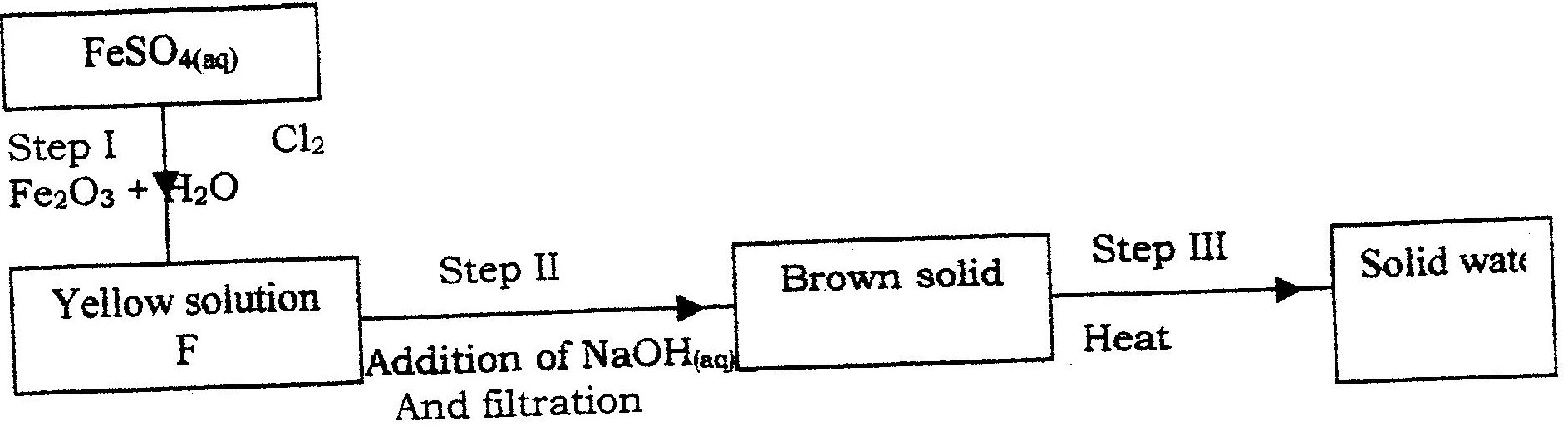
Potassium sulphite solution was prepared and divided into two portions. The first portion gave a white precipitate when reacted with barium nitrate. On addition of dilute hydrochloric acid the white precipitate disappeared.
a) Write the formula of the compound which formed as the white precipitate. b) Write the equation for the reaction between dilute hydrochloric acid and the compound whose formula is written in (a) above. c) What observation would be made if one drop of potassium dichromate solution was added to the second portion followed by dilute hydrochloric acid?
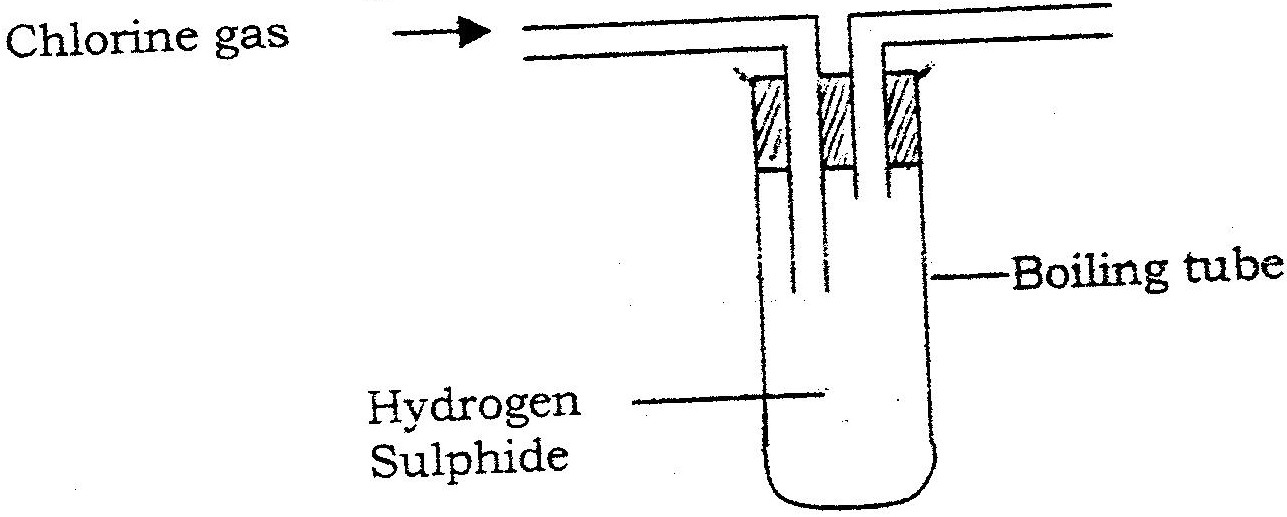
In an experiment, chlorine gas was passed into moist hydrogen sulphide contained in a boiling tube as shown in the diagram
a) What observation was made in the boiling tube?
b) Write an equation for the above reaction. c) What precaution should be taken in carrying out this experiment? Give a reason.
Explain why anhydrous magnesium chloride is fairly soluble in organic solvents while anhydrous magnesium chloride is insoluble.
Expected Response
Aluminum chloride is covalent while magnesium chloride is ionic
0.63g of lead powder were dissolved in excess nitric acid to form lead nitrate solution. All the lead nitrate solution was reacted with sodium sulphate solution.
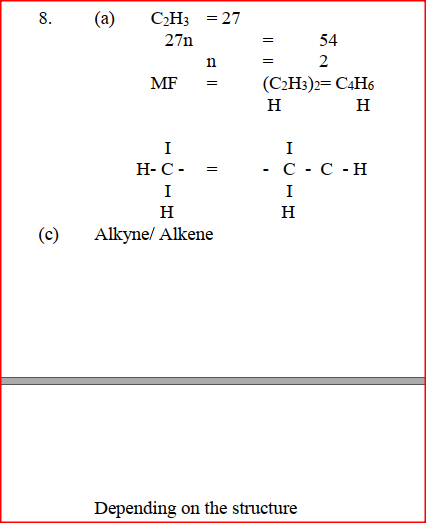
The empirical formula of a hydrocarbon is C2H3.The hydrocarbon has a relative molecular mass of 54..(H = 1.0, C = 12.0). a) C2H3 b) Draw the structural formula of the hydrocarbon c) To which homologous series does the hydrocarbon drawn in (b) above belong?
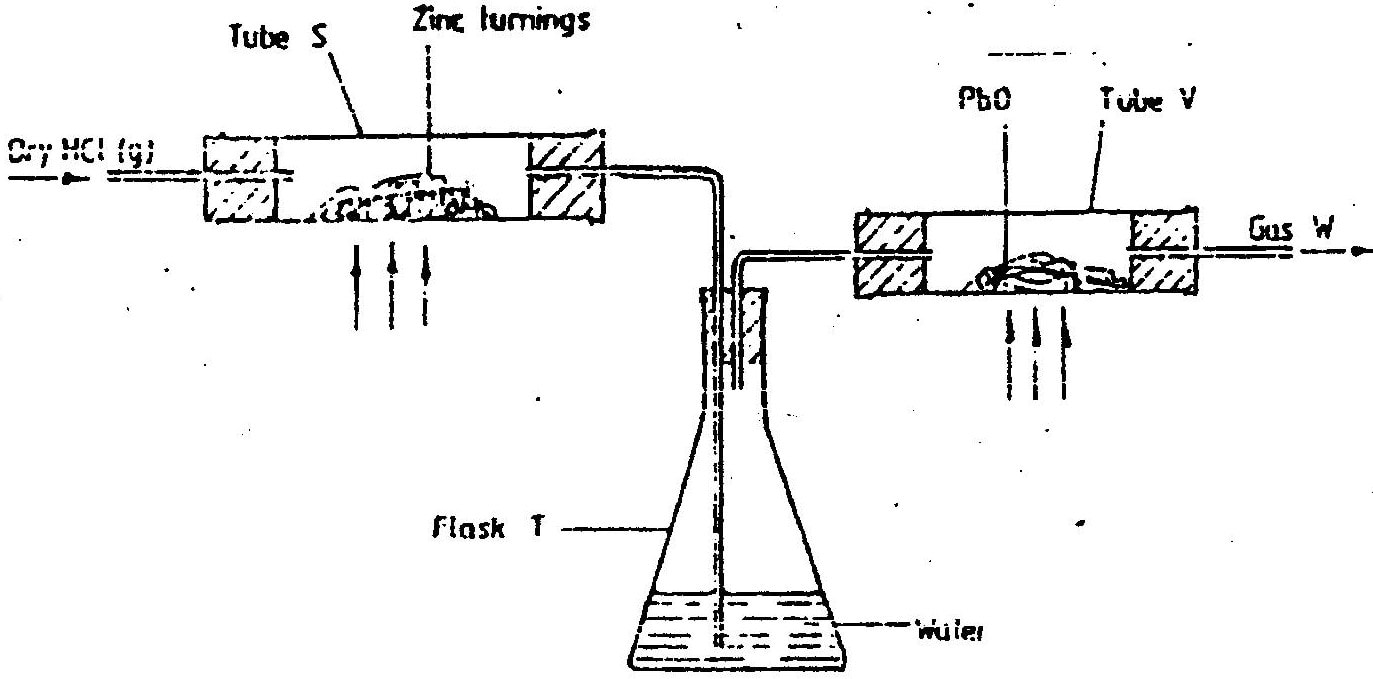
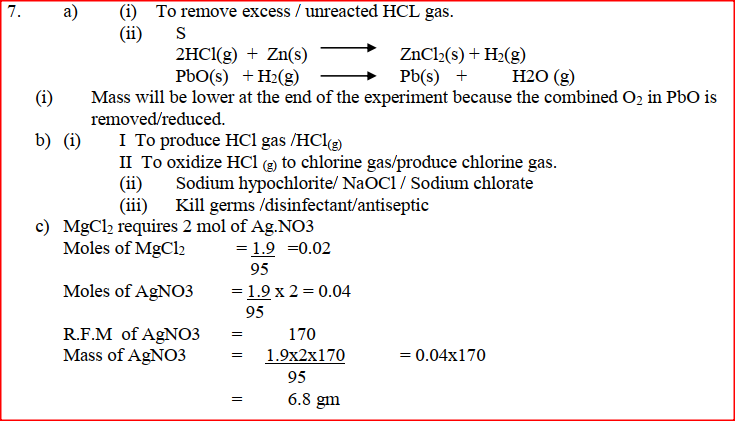
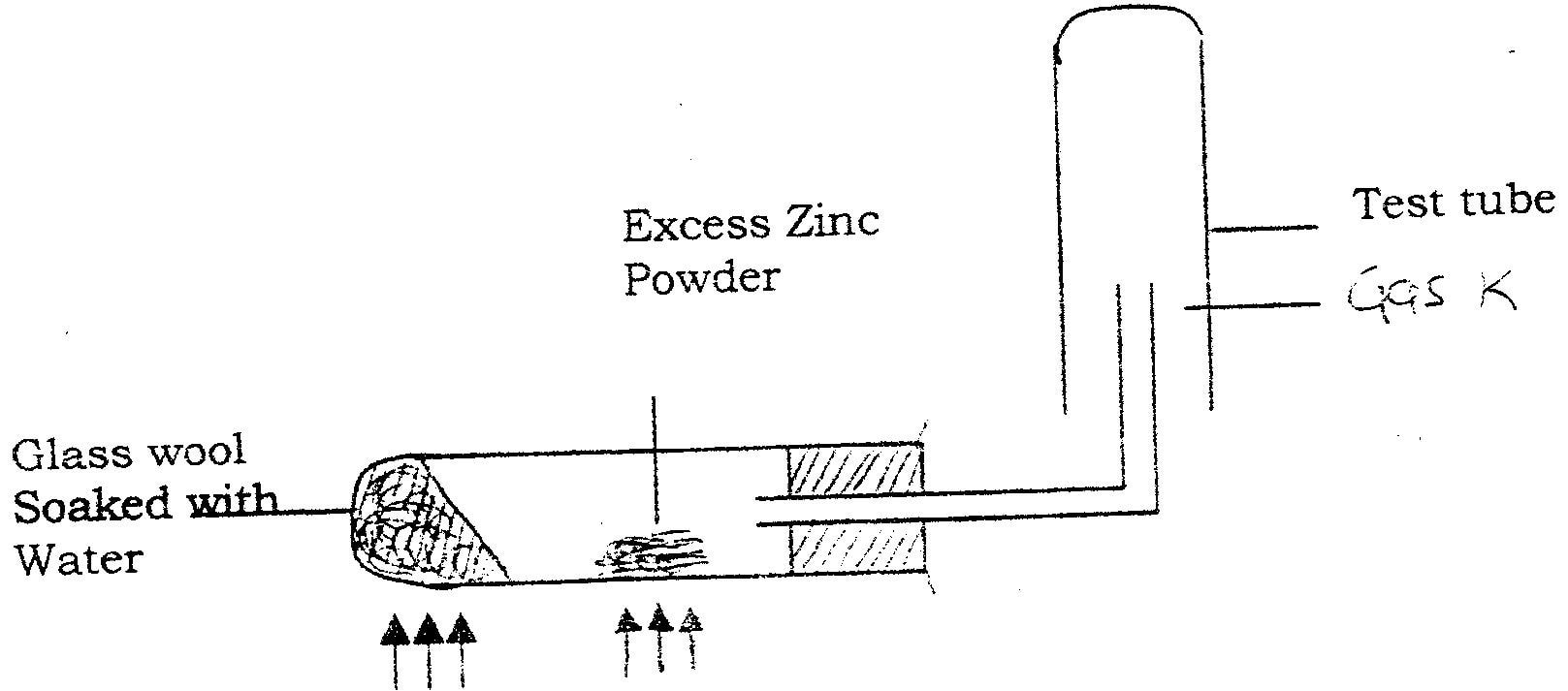
(a) In an experiment, dry hydrogen chloride gas was passed through heated zinc turnings as shown in the diagram below. The gas produced was then passed through heated lead (II) oxide.
Calculate the mass of nitrogen dioxide gas that would occupy the same volume as 10g of hydrogen gas at same temperature and pressure.(H = 1.0, N = 14.0, o = 16.0)
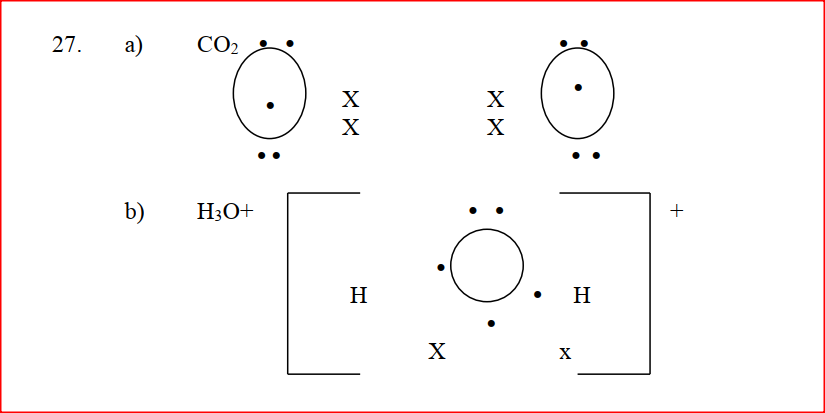
Using dots (.)and crosses (x) to represent outermost electrons, draw diagrams to show the bonding in CO2 and H3O+ (Atomic numbers; H = 1.0,C= 14.0, O = 8 ).
The information below relates to element L, Q, R and T. The letters do not represent the actual symbols of the elements. Arrange the elements in
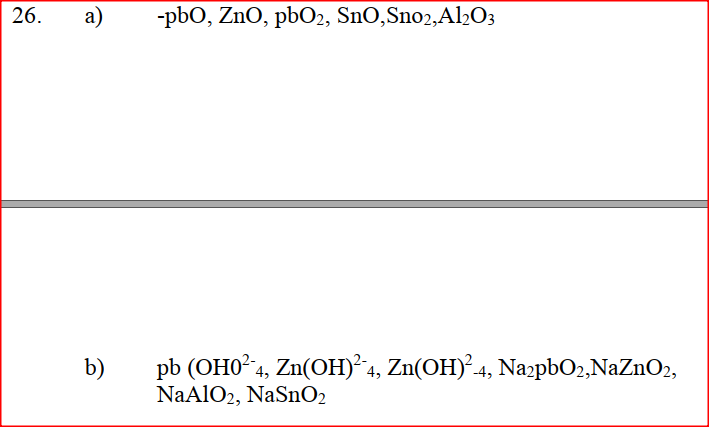
a) Give the formula of an oxide which reacts with both dilute hydrochloric acid and hot concentrated sodium hydroxide. b) Give the formulae of the products formed when the oxide in (a) above reacts with excess hot concentrated sodium hydroxide. |
Chemistry Topics
All
Archives
December 2024
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
Can't find what you want? Use this Search box. |
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |

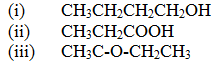

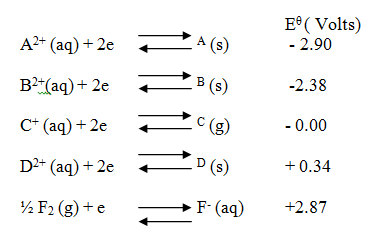







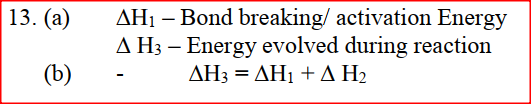

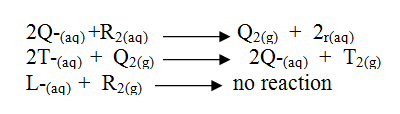




 RSS Feed
RSS Feed

