|
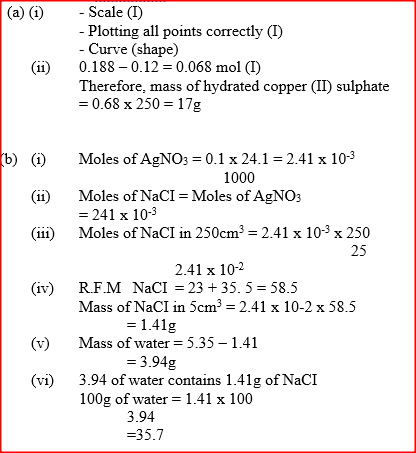
(a) The table below gives the solubilities of hydrated copper (II) sulphate in mol dm3 at different temperatures (i) On the grid provided, plot a graph of solubility of copper (II) sulphate (vertical axis) against temperature. (ii) From the graph, determine the mass of copper (II) sulphate deposited when solution is cooled from 700C to 400C. (Molar mass of hydrated copper (II) sulphate = 250g) (b) In an experiment to determine the solubility of sodium chloride, 5.0cm3 of a saturate solution of sodium chloride weighing 5.35g were placed volumetric and diluted to a total volume of 250cm3. 25.0cm3 of the dilute solution chloride completely reacted with 24cm3 of 0.1M silver nitrate solution. AgNO3(aq) + NaCI(aq) → AgCl(s) + NaNO3(aq) Calculate: (i) Moles of silver nitrate in 24cm3 of solution (ii) Moles of sodium chloride in 25.0cm3 of sodium (iii) Moles of sodium chloride in 250cm3 of solution (iv) Mass of sodium chloride in 5.0cm3 of saturated sodium chloride Solution (Na = 23.0, CI = 35.5) (v) Mass of water in 5.0cm3 of saturated solution of sodium chloride (vi) The solubility of sodium chloride in g/100 water
0 Comments
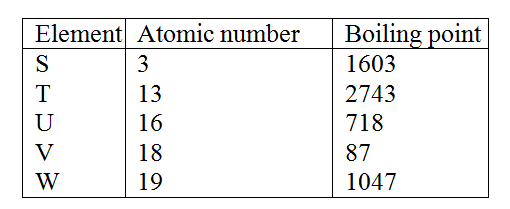
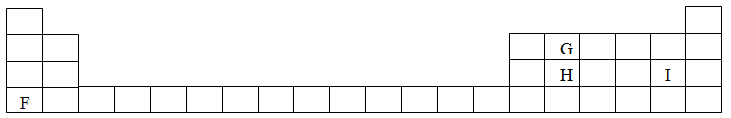
Study the information given in the table below and answer the questions that follow. The letters do not represents the actual symbols of the elements
(a) Select the elements which belong to the same
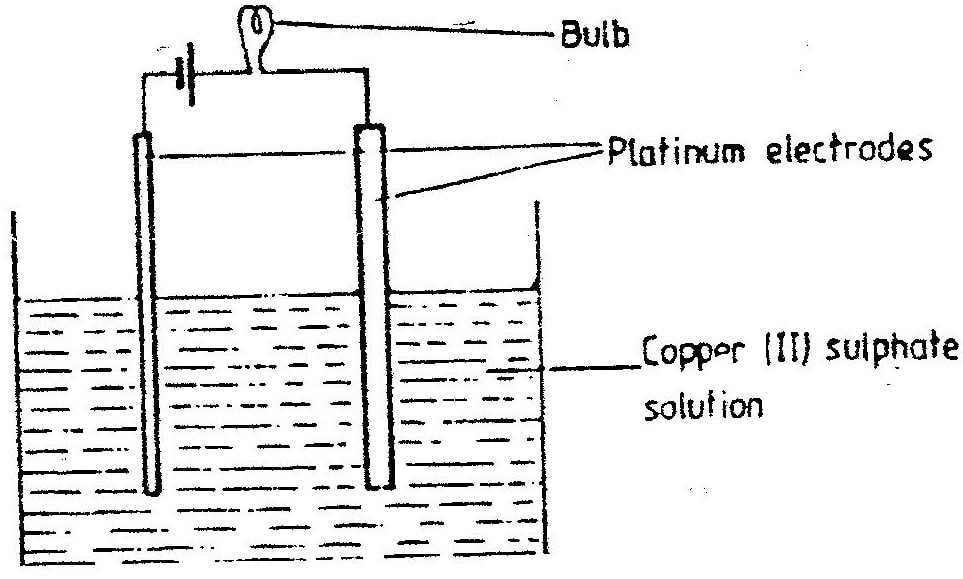
(e) The aqueous sulphate of element W was electrolyzed using inert electrodes Name the products formed at the:
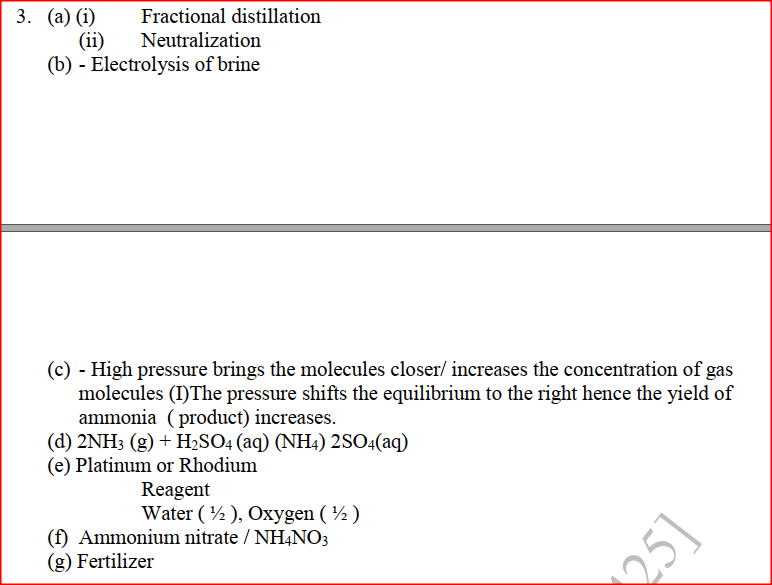
The flow chart below shows the industrial preparation of ammonia and the process used in the manufacture of some ammonium compounds. Study and answer the questions that follow
(a) Give the name of the
(c) Explain why it necessary to compress nitrogen and hydrogen in this process (d) Write an equation for the reaction which takes place in step 6 (e) Name the catalyst and the reagents used in step 3 Catalyst Reagent (f) Name compound Z1 (g) Give one commercial use of compound Z2
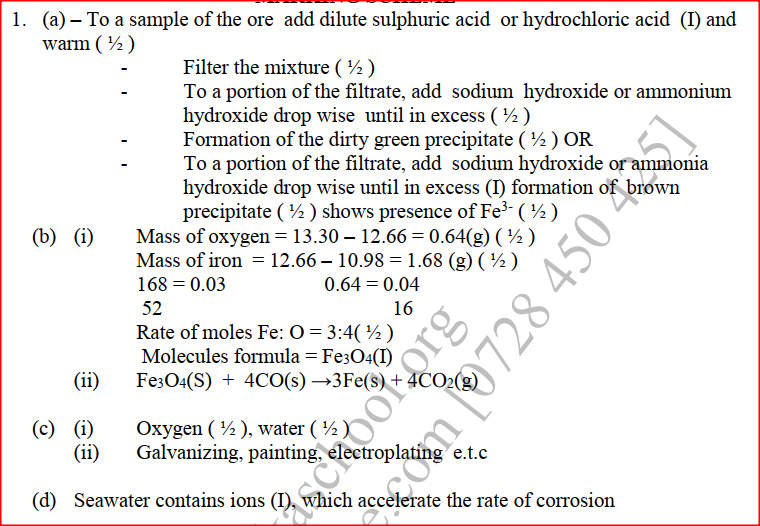
(a) An ore is suspected to contain mainly iron. Describe a method that can be used to confirm the presence of iron in the ore
(b) Excess carbon monoxide gas was passed over a heated sample of an oxide of iron as shown in the diagram below. Study the diagram and the data below it to answer the questions that follow.
Mass of empty dish = 10.98g
Mass of empty dish 4 oxide of iron = 13.30g Mass of empty dish 4 residue = 12.66g
(c) Corrosive is a destructive process in which iron which is converted into hydrated iron (III) oxide State:
(d) Explain why it is not advisable to wash vehicles using seawater
(a) The following equations represents two different types of reactions
(i) nC4H8(g) → (C4H8)n(s) (ii) C2H6(g) + CI2(g) → C2H5CI(I) + HCI(g) State the type of reaction represented by: (i) (ii) (b) The fermentation of glucose produces ethanol as shown in the equation below C2H12O6(aq) → 2CH3CH2OH(aq) + 2CO2(g)
(c) The molecular formula of a hydrocarbon is C6H14. The hydrocarbon can be converted into two other hydrocarbons as shown by the equation below (i) Name and draw the possible structural formula of X Name Structural formula (ii) State and explain the observation that would be made if a few drops of bromide water were added to a sample of X. (iii) Write an equation for the complete combustion of C3H8
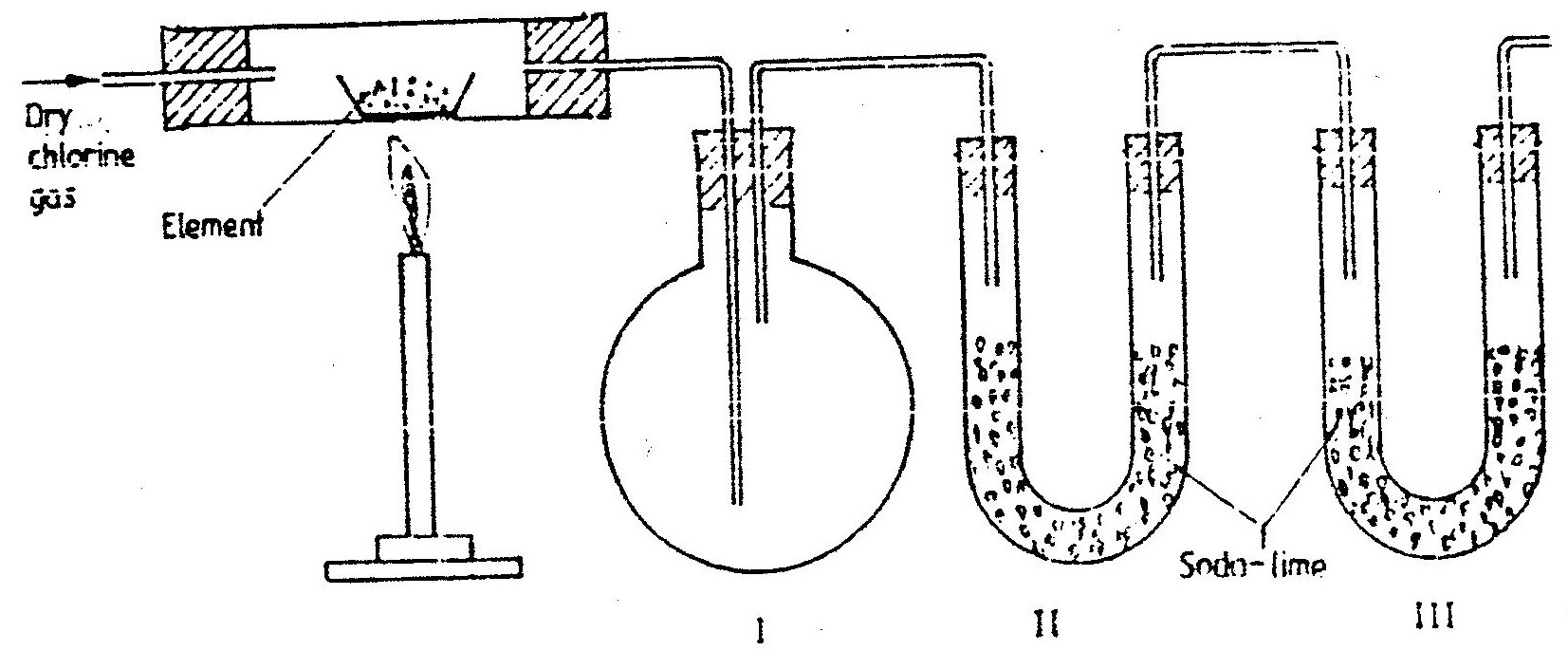
The set – up below was used to prepare anhydrous chlorides of a number of elements in a laboratory where no fine cupboard was available. The chlorides were to be collected in flask
The following table shows the melting and boiling points of the chlorides that were prepared.
More Quiz
(a) The table below gives the solubilities of hydrated copper (II) sulphate in mol dm3 at different temperatures (i) On the grid provided, plot a graph of solubility of copper (II) sulphate (vertical axis) against temperature. (ii) From the graph, determine the mass of copper (II) sulphate deposited when solution is cooled from 700C to 400C. (Molar mass of hydrated copper (II) sulphate = 250g) (b) In an experiment to determine the solubility of sodium chloride, 5.0cm3 of a saturate solution of sodium chloride weighing 5.35g were placed volumetric and diluted to a total volume of 250cm3. 25.0cm3 of the dilute solution chloride completely reacted with 24cm3 of 0.1M silver nitrate solution. AgNO3(aq) + NaCI(aq) → AgCl(s) + NaNO3(aq) Calculate: (i) Moles of silver nitrate in 24cm3 of solution (ii) Moles of sodium chloride in 25.0cm3 of sodium (iii) Moles of sodium chloride in 250cm3 of solution (iv) Mass of sodium chloride in 5.0cm3 of saturated sodium chloride Solution (Na = 23.0, CI = 35.5) (v) Mass of water in 5.0cm3 of saturated solution of sodium chloride (vi) The solubility of sodium chloride in g/100 water In order to determine the molar of neutralization of sodium hydroxide, 100cm3 of 1M sodium hydroxide and 100cm3 of 1 M hydrochloric acid both at the same initial temperature were mixed and stirred continuously with a thermometer. The thermometer of the resulting solution was recorded after every 30 seconds until the highest temperature of the solution was attained. Thereafter the temperature of the solution was recorded for a further two minutes
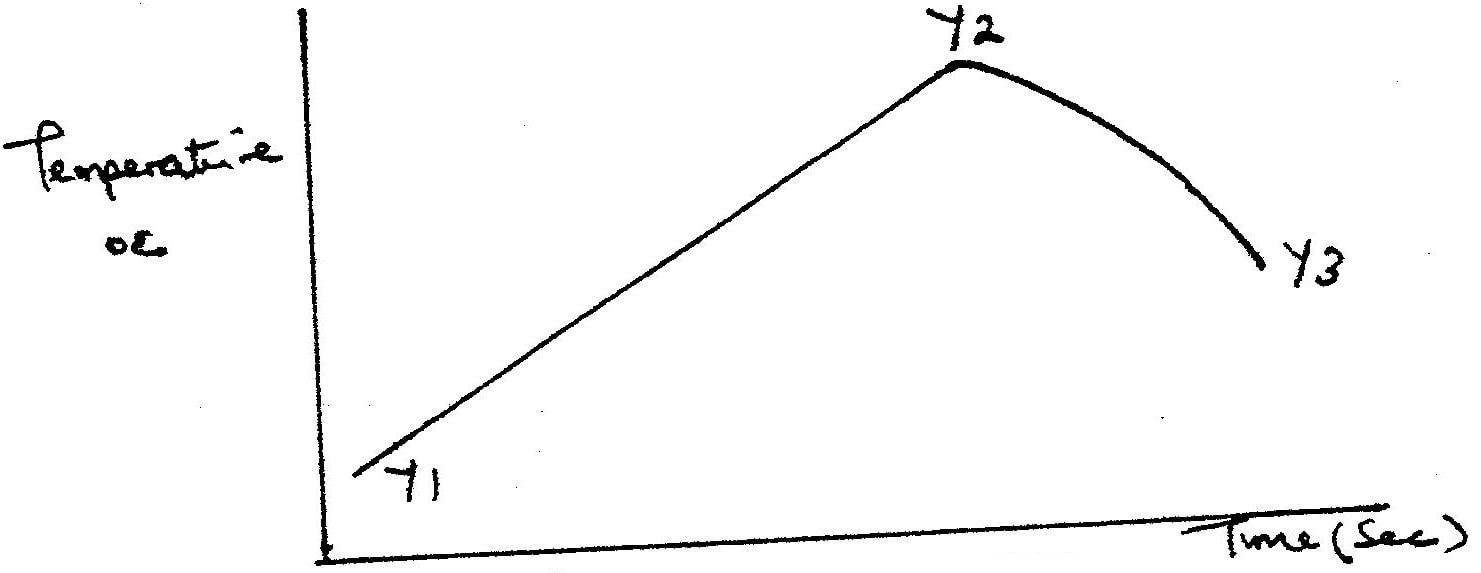
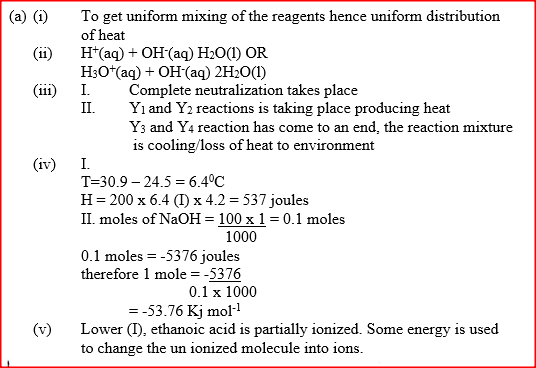
(a)
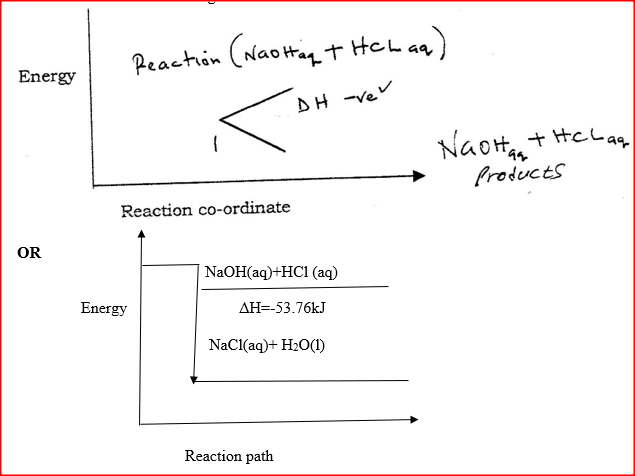
I. What is the significance of point Y2? II. Explain why there is a temperature change between points; Y1 and Y2 Y3 and Y4 (iv) In the initial temperature for both solutions was 24.50C and the highest temperature attained by the mixture was 30.90C Calculate the: I. heat change for the reaction (specific heat capacity of the solution = 4.2Jg -1K-1 and the density of the solution = 1.0g/cm3 II. Molar heat of neutralization of sodium hydroxide (v) Explain how the value of the molar heat of neutralization obtained in this experiment would compare with the one that would be obtained if the experiment was repeated using 100cm3 of 1 Methanoic acid instead of hydrochloric acid. (b) On the grid provided below, draw an energy level diagram for the reaction between hydrochloric acid and sodium hydroxide What is the oxidation number of chlorine in CO4
Expected Response
The column below was used do soften hard water
Expected Response
A hydrocarbon slowly decolorizes bromine gas in the presence of sunlight but does not decolourise acidified potassium permanganate
Name and draw the structural formula of the fourth member of the series to which the hydrocarbon belongs.
Expected Response
The table below gives the energy required to remove the outermost electron for some group I elements
Arrange the elements in order of their reactivity starting with the most reactive
Expected Response
The diagram below shows the physical state of matter. Study it and answer the questions that follow
Identify the processes R, V, w and U
(c) Name one substance which can undergo the process represented by S and T.
Expected Response
A beekeeper found that when stung by a bee, application of a little solution of hydrogen carbonate helped to relieve the irritation from the affected area. Explain.
Expected Response
A sealed glass tube containing air at s.t.p was immersed in water at 1000c. Assuming that there was no increase in the volume of the glass tube due to the expansion of the glass, calculate the pressure of the inside tube.
(standard pressure = 760mmHg,)
Expected Response
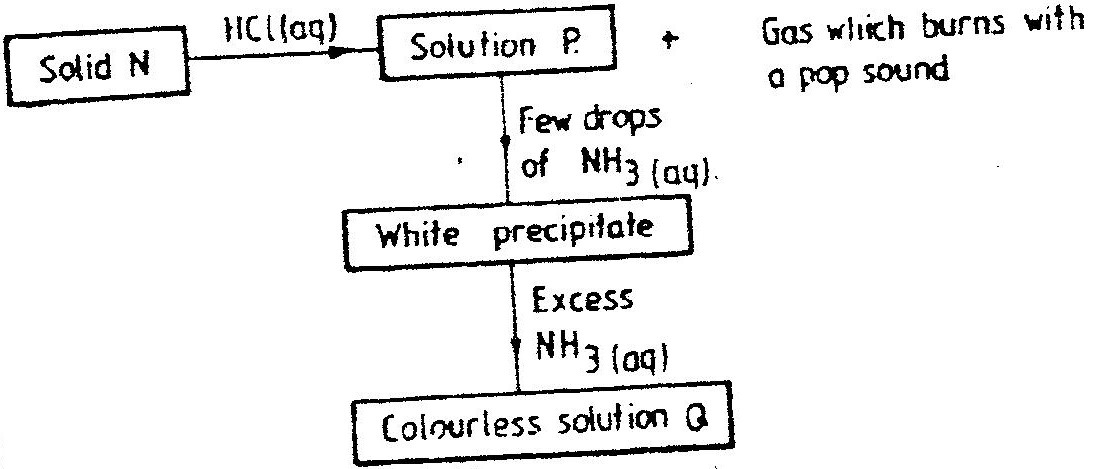
The scheme below shows some reaction sequence starting with solid N.
Expected Response
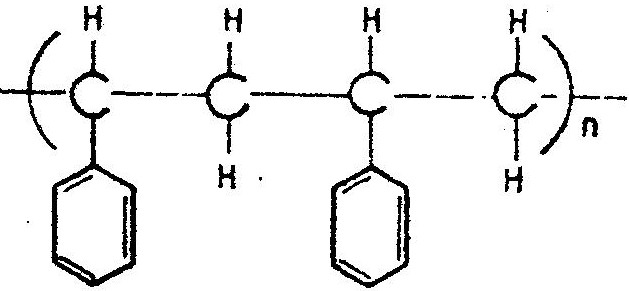
The formula given below represents a portion of a polymer Give:
Expected Response
The diagram below represents a charcoal burner. Study it and answer the questions that follow
Write equations for the reactions taking place at I and I and II
Expected Response
Describe how a solid sample of lead (II) Chloride can be prepared using the following reagents, dilute nitric acid, dilute hydrochloric acid and lead carbonate.
Expected Response
Urea, (NH2)2CO is prepared by the reaction between ammonia and carbon dioxide. 2NH3(g) + CO2(g) → (NH2)2CO(aq) + H2O(l). In one process, 680 kg of ammonia were reacted with excess carbon dioxide. Calculate the mass of urea that was formed. (H = 1.0, c+ 12.0, N =14.0, O = 16.0 and relative molecular mass of ammonia = 17) Calculate the mass of Urea that was formed
Expected Response
Expected Response
Expected Response
The grid below shows part of a periodic table. The letters do not represent the actual symbols of the elements
Expected Response
Draw the structural formula of:
Expected Response
Distinguished between a strong and a weak acid. Give examples.
|
Chemistry Topics
All
Archives
December 2024
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
|
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |




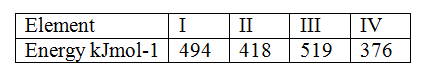


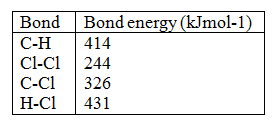



 RSS Feed
RSS Feed

