|
Diamond and graphite are allotropes of carbon. In terms of structure and bonding explain the following.
0 Comments
A sample of water drawn from a river passing through an agricultural district was divided into two portions. The first portion gave a white precipitate when acidified barium chloride was added. The second portion when warmed with aqueous sodium hydroxide gave a colourless gas, which turned a moist red litmus paper blue.
On complete combustion of a sample of hydrocarbon, 3.52 gm of carbon dioxide and 1.44 gm of water were formed. Determine the molecular formula of the hydrocarbon.
(Relative molecular masses of hydrocarbon = 56, carbon dioxide 44, water = 18 and relative atomic masses H = 1.0 and c = 12.0)
Give one advantage and one disadvantage of using petrol containing tetraethyl lead in motor vehicles.
Expected Response
Advantage
Expected response
Add water to the solid mixture A dissolves while B does Not
An isotope of Uranium 234U, decays by emission of an alpha particle to thorium 92
|
Chemistry Topics
All
Archives
December 2024
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
|
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |









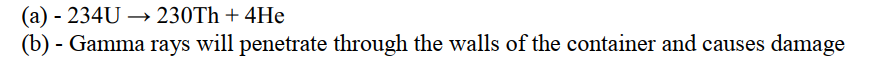

 RSS Feed
RSS Feed

