|
(a) A student was supplied with a colourless liquid suspected to be water
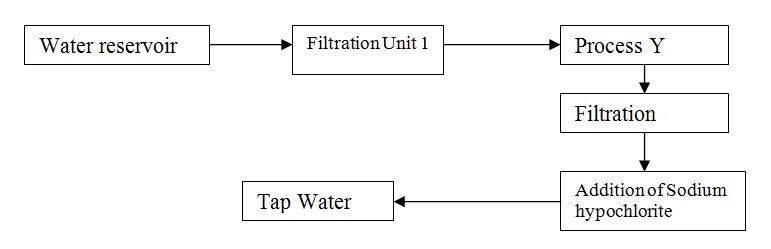
The flow chart below shows the various stages of water treatment. Study it and answer the questions that follow
II Addition of sodium hypochlorite (c) It was confirmed that magnesium sulphate was present in the tap water
0 Comments
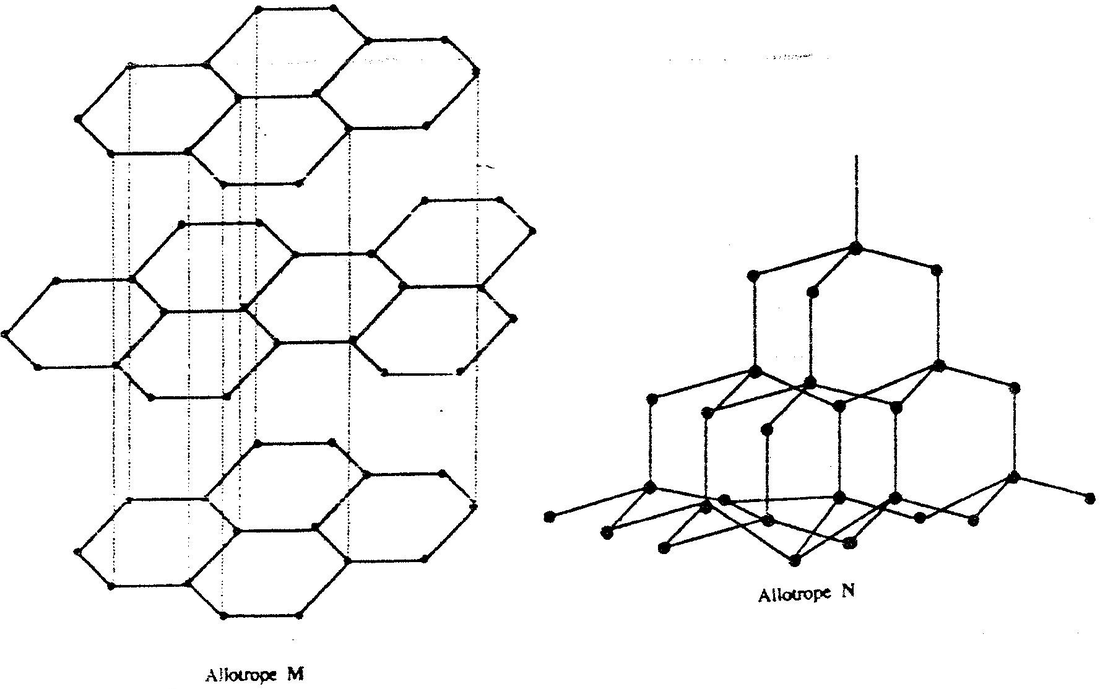
(a) The following diagrams show the structures of two allotropes of carbon. Study them and answer the questions that follow
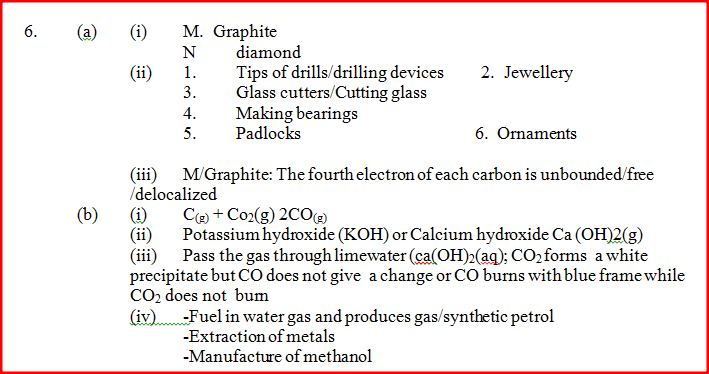
(i) Name allotrope
M N (ii) Give one use of N (iii) Which allotrope conducts electricity? Explain
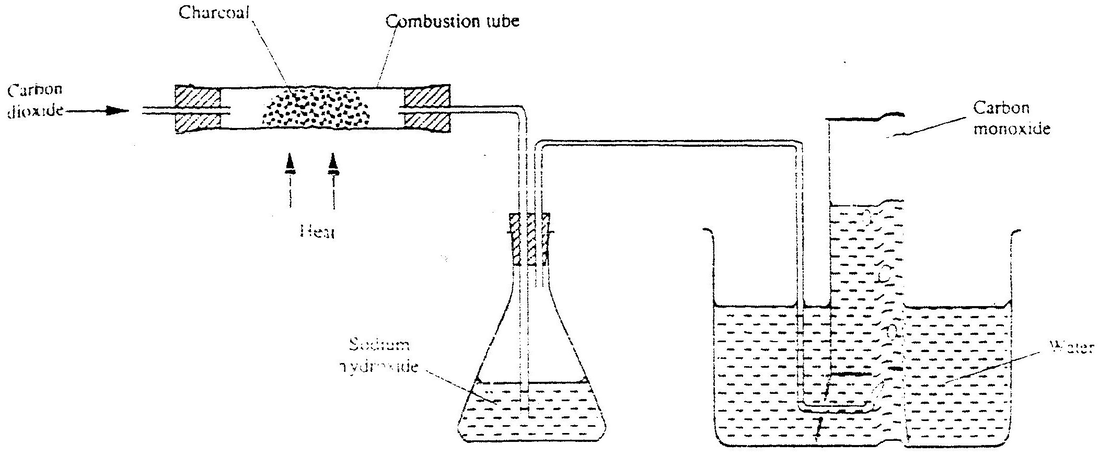
(b) In an experiment, carbon dioxide gas as passed over heated charcoal and the gas produced collected as shown in the diagram below
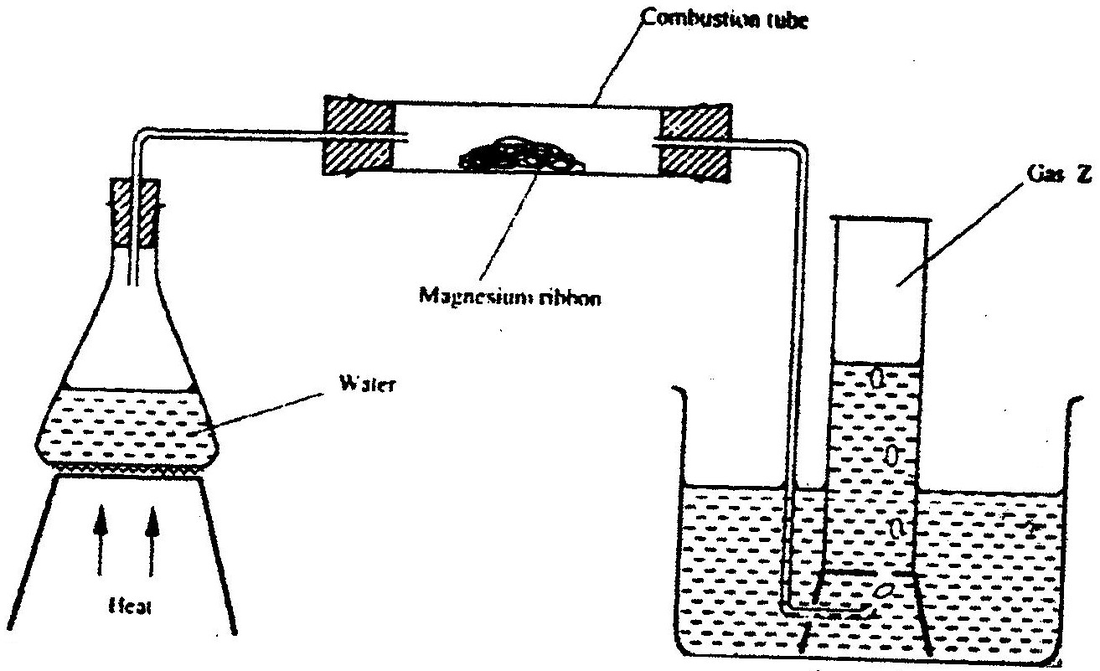
(i) Write an equation for the reaction that took place in the combustion tube
(ii) Name another substance that can be used instead of sodium hydroxide (iii) Describe a sample chemical test that can be used to distinguish between carbon dioxide and carbon monoxide (iv) Give one use of carbon monoxide
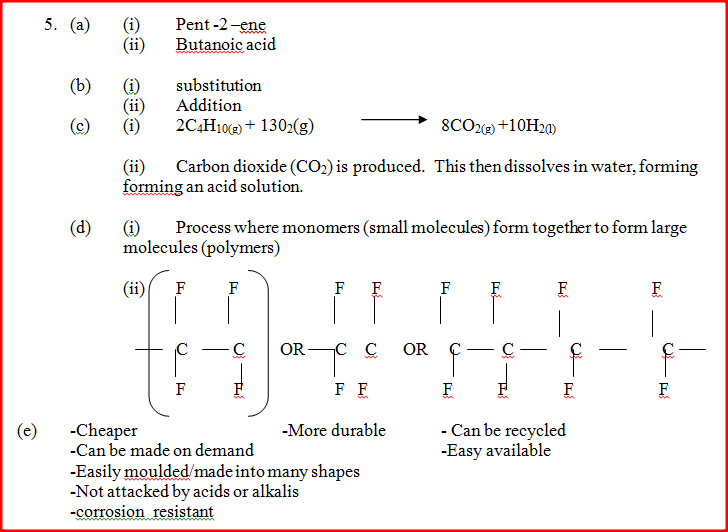
(a) Give the names of the following compounds
Name the type of bromination reaction that takes place in: (i) and (ii)
(d) The polymerization of tetra flouroathene (C2H4) is similar to that of ethane ( C2H4)
(e) State any two advantages that synthetic polymers have over natural polymers
(a)
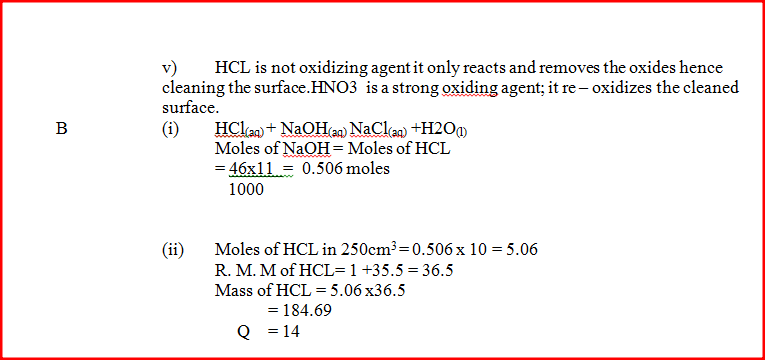
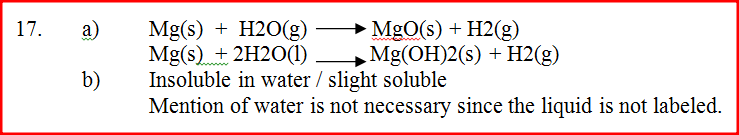
(b) A sample of hydrogen chloride gas was dissolved in water to make 250cm3 of solution required 46 cm3 of 11.0M Sodium hydroxide for complete neutralization.
State and explain the function of tartaric acid in baking powder
Expected Response
It reacts with NaHCO3 to form CO2 which causes the dough to rise.
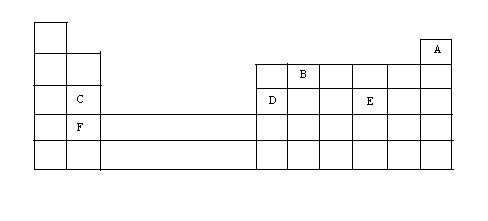
(a) The grid given below represents part of the periodic table. Study it and answer the questions that follow. (the letters do not represents the actual symbol of the elements)
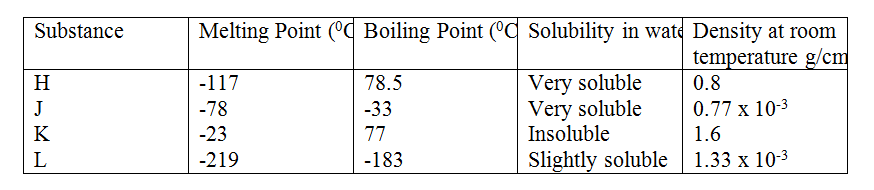
(b) Study the information in the table below and answer the questions that follow (the letters do not represent the actual symbol of the substances)
II. By downward displacement of air? (Density of air is 1.29 x 10-3g/cm3 at room temperature. Related Chemistry Questions and Answers on Chemical Families Form 2 Level
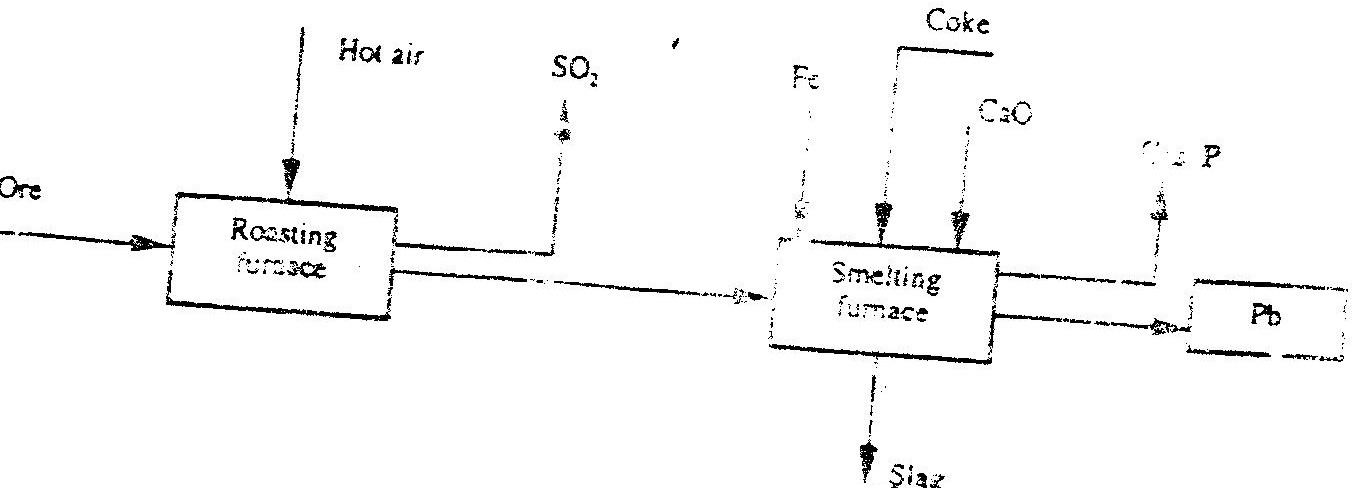
The flow chart below illustrate the industrial extraction of lead metal. Study it and answer the questions that follow
(a)
(c) State one use of lead other than the making of lead pipes Related Chemistry Questions and Answers on Metals Form 4 Level
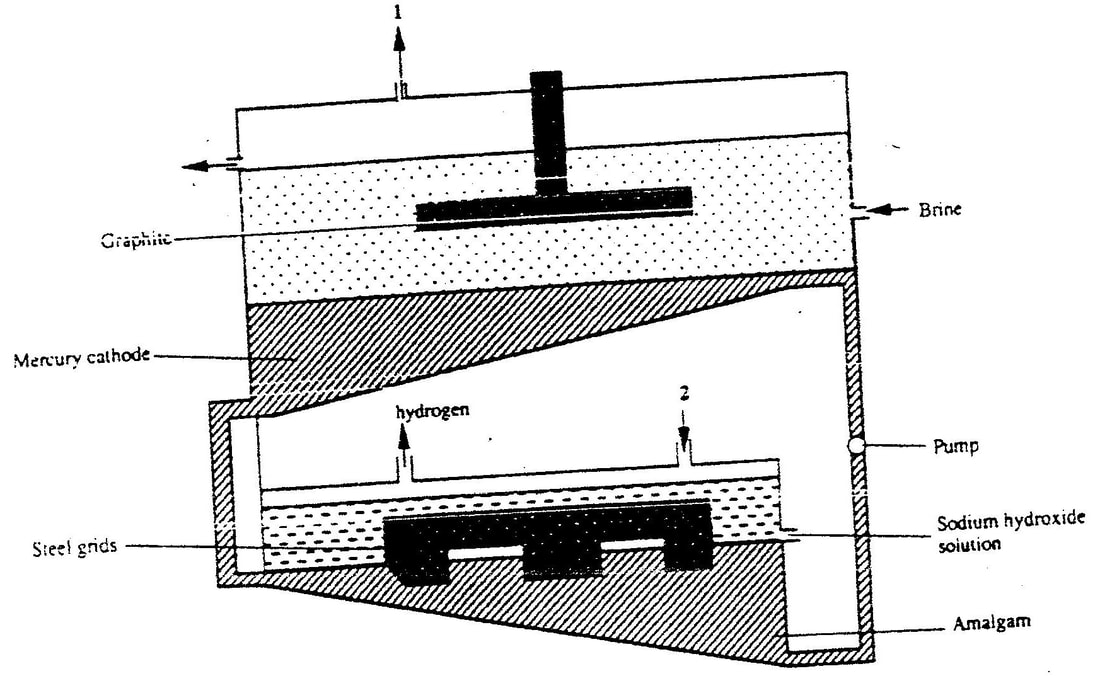
1 (a) The diagram below represents a mercury cell that can be used in the industrial manufacture of sodium hydroxide. Study it and answer the question that follow
In an experiment, ammonia chloride was heated in a test tube. A moist red litmus paper placed at the mouth of the test tube first changed blue then red. Explain these observations
Expected Response
NH4Cl decomposes to form NH3(g) and HCl(g).Ammonia diffuses faster than HCl because its light. Ammonia is basic and thus red litmus paper turns blue while HCl is acid thus blue litmus turns red.
Explain why it is not advisable to leave a Jiko with burning charcoal in a closed room where one is sleeping.
Expected Response
The supply of oxygen in the room will be limited leading to formation of CO which is poisonous.
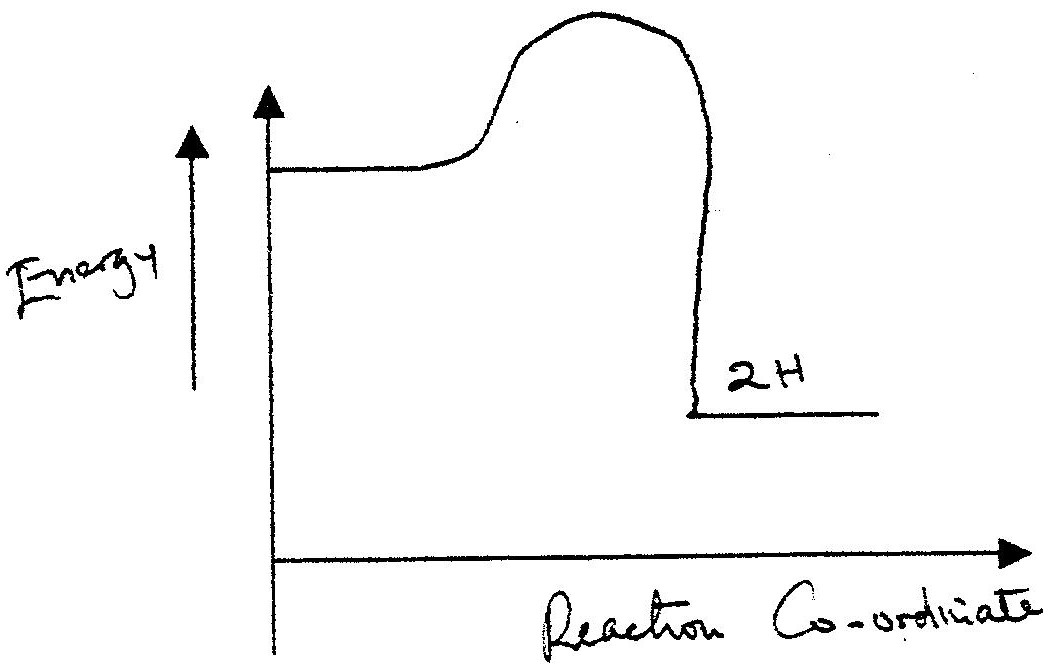
Hydrogen and fluorine react according to the equation below
When a solid sample of sulphur is heated in a test tube, it changes into a liquid, which flows easily. On further heating, the liquid darkness and does not flow easily. Explain these observations.
Expected Response
Solid sulphur is made of S8 rings. It melts into aliquid of S8 rings, On further heating the rings open up to form long chains of sulphur atoms, which then entangle making it viscous and dark, or sulphur melts into S8 molecules. The molecules join up to form long chain which entangle making it viscous and dark
Study the information in the table below and answer the questions that follow. The letters do not represent the actual symbols of the elements)
Select an element which
Expected Response
Name another gas, which is used together with oxygen in welding
Expected Response
Acet5ylene (ethyne) or Hydrogen
Pentane and ethanol are miscible. Describe how water could be used to separate a mixture of pentane and ethanol
Expected Response
20. Add water to the mixture in a separating funnel. Ethanol dissolves while pentane does not. Allow the mixture to separate in two layers. Open the tap to drain the lower aqueous layer. Distil the water ethanol mixture to get ethanol.
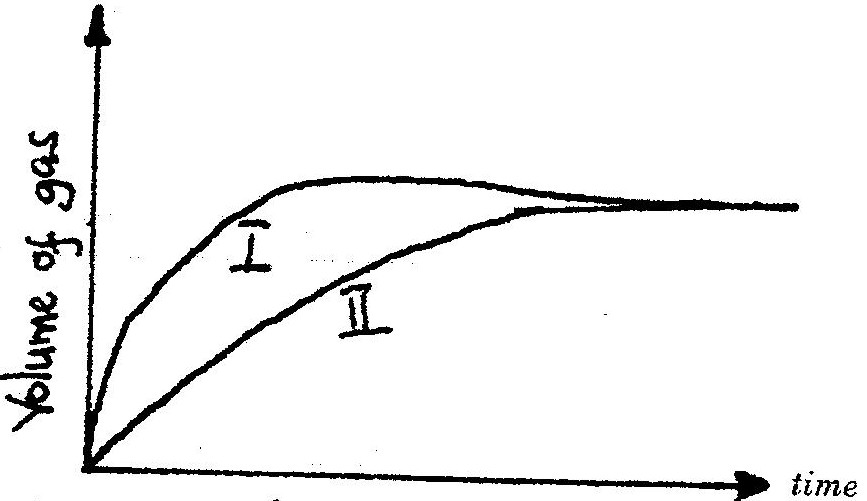
The curves below were obtained when two equal volumes of hydrogen peroxide of the same concentration were allowed to decompose separately. In one case, manganese (IV) oxide was added to the hydrogen peroxide
Which curve represents the decomposition of hydrogen peroxide with manganese (IV) oxide? Explain
Expected Response
19. I – Manganese (iv) Oxide is a catalyst and increases the rate of decomposition of the hydrogen peroxide.
A given volume of ozone, (O3) diffused from a certain apparatus in 96 seconds. Calculate the time taken by an equal volume of carbon dioxide (CO2) to diffuse under the same conditions (O = 16.0, C = 12.0)
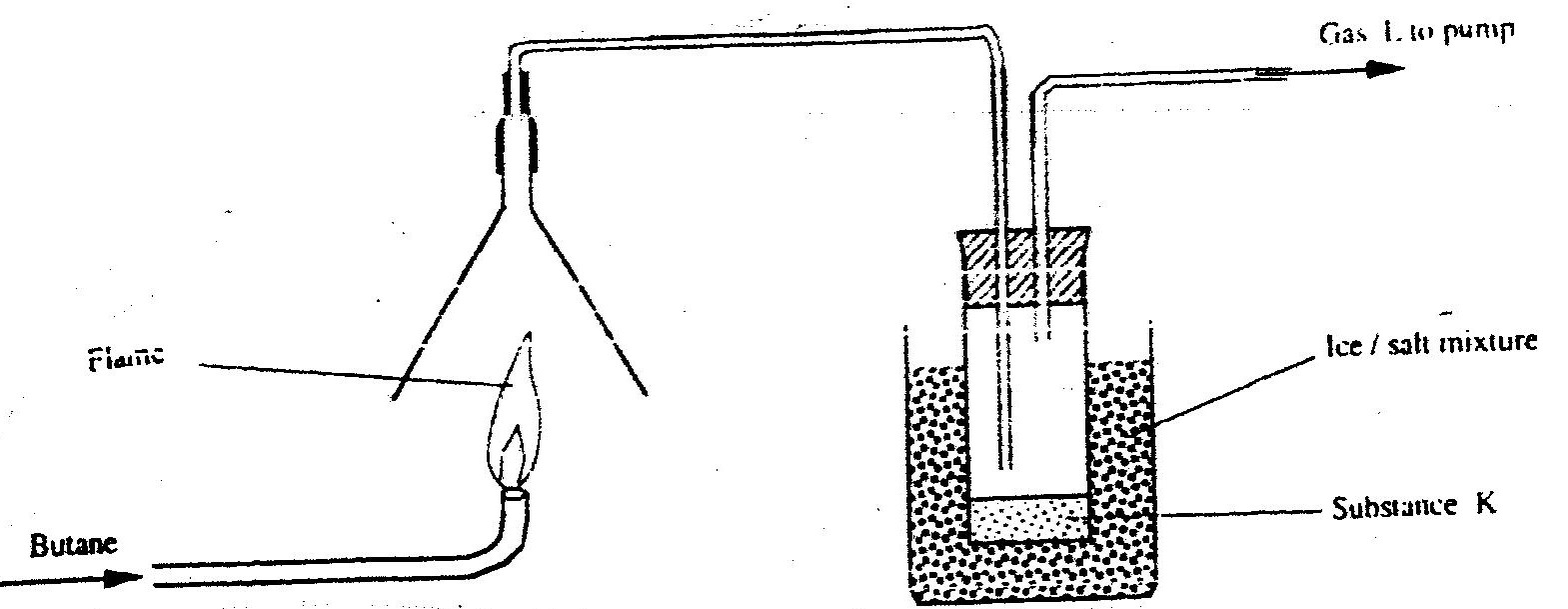
Study the set- up below and answer the questions that follow
(a) Write an equation for the reaction, which take place in the combustion tube
(b) What property of gas Z allows it to be collected as shown in the diagram
Compound Q is a solid with a giant ionic structure. In what form would the compound conduct an electric current
Expected Response
16. When dissolves in water or fused / molten state
State any two differences between luminous and non – luminous flames
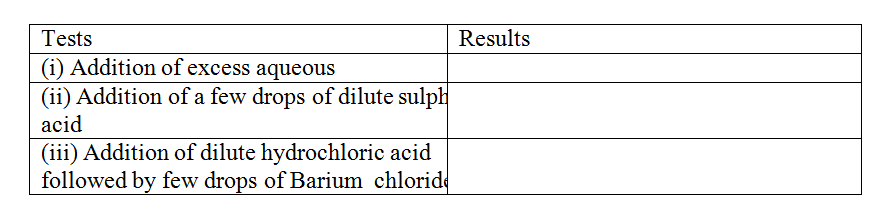
The table below shows the tests carried out on separate samples of water drawn from a well and the results obtained
(a) Identify the cation and the anion present in the water
Cation Anion (b) Write an ionic equation for the reaction which takes place in test (iii) A radioactive isotope X2 decays by emitting two alpha (a) particles and one beta (β) to from 214 Bi 83 (a) What is the atomic number of X2? (b) After 112 days, 1/16 of the mass of X2 remained. Determine the half life of X2
Expected Response
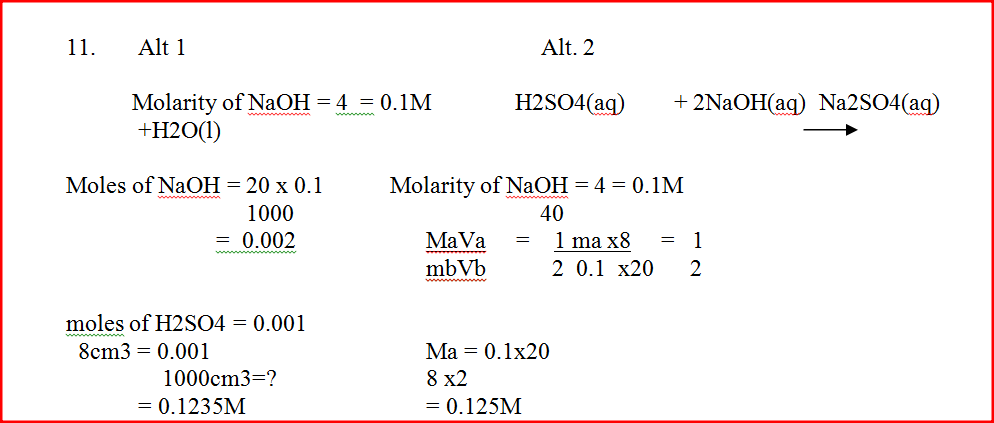
20.0cm3 of a solution containing 4 gm per litre of sodium hydroxide was neutralized by 8.0cm3 of dilute sulphuric acid. Calculate the concentration of sulphuric acid in moles per litre (Na = 23.0, O = 16.0, H = 1.0)
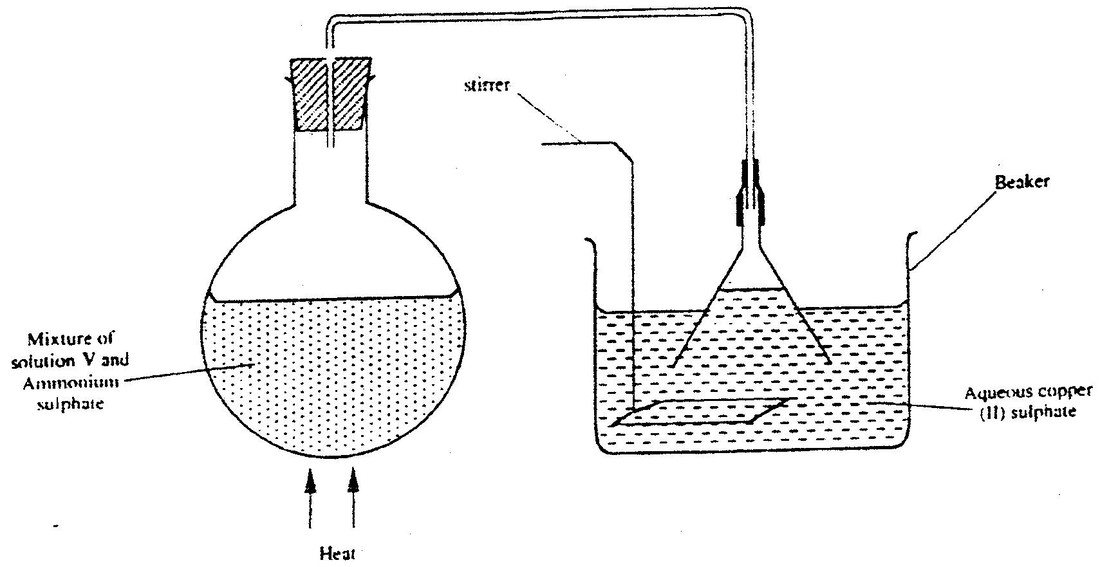
A student set up the apparatus shown below to prepare ammonia gas and react it with copper (II) sulphate solution
(a) Identify solution V
(b) State the observations which were made in the beaker
Expected Response
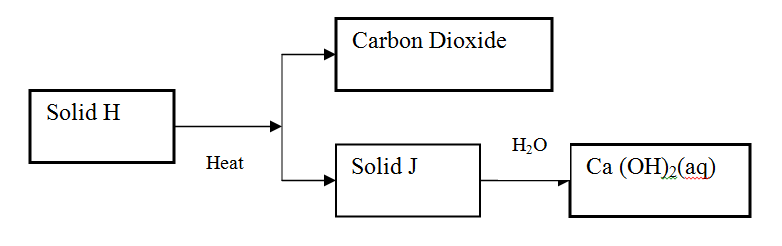
Use the scheme below to answer the questions that follow
(a) Identify the solid
H J (b) State one commercial use of solid J |
Chemistry Topics
All
Archives
December 2024
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
Can't find what you want? Use this Search box. |
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |




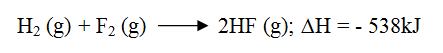

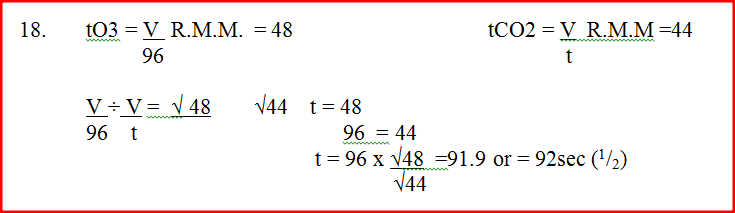
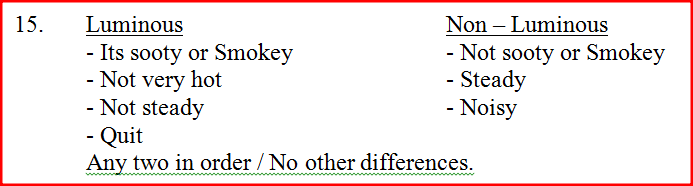
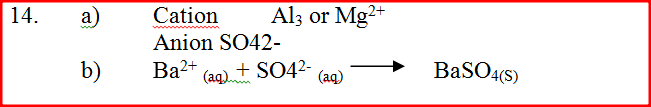


 RSS Feed
RSS Feed

