|
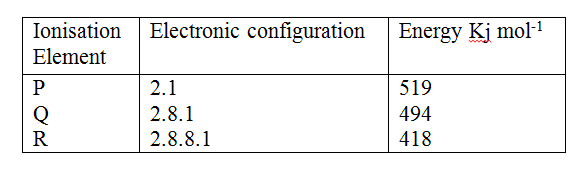
a) Study the information in the table below and answer the questions that follow.
(The letters do not represent the actual symbols of the elements).
c) Neutralization is one of the methods of preparing salts. i) What is meant by neutralization? ii) Describe how you would prepare crystals of sodium nitrate starting with 200cm3 of 2M sodium hydroxide. iii) Write an equation for the reaction that takes place when a solid sample of sodium nitrate is heated.
0 Comments
(Fe - = 56.0,Cl = 35.5 and Molar gas volume at 298K is 24,000cm3
C) When hydrogen sulphide gas was passed through a solution of iron (III) chloride, the following observation were made:
a) Fraction distillation of liquid air usually produces nitrogen and oxygen as the major products.
(Boiling points nitrogen = - 1960C, oxygen = -1830C)
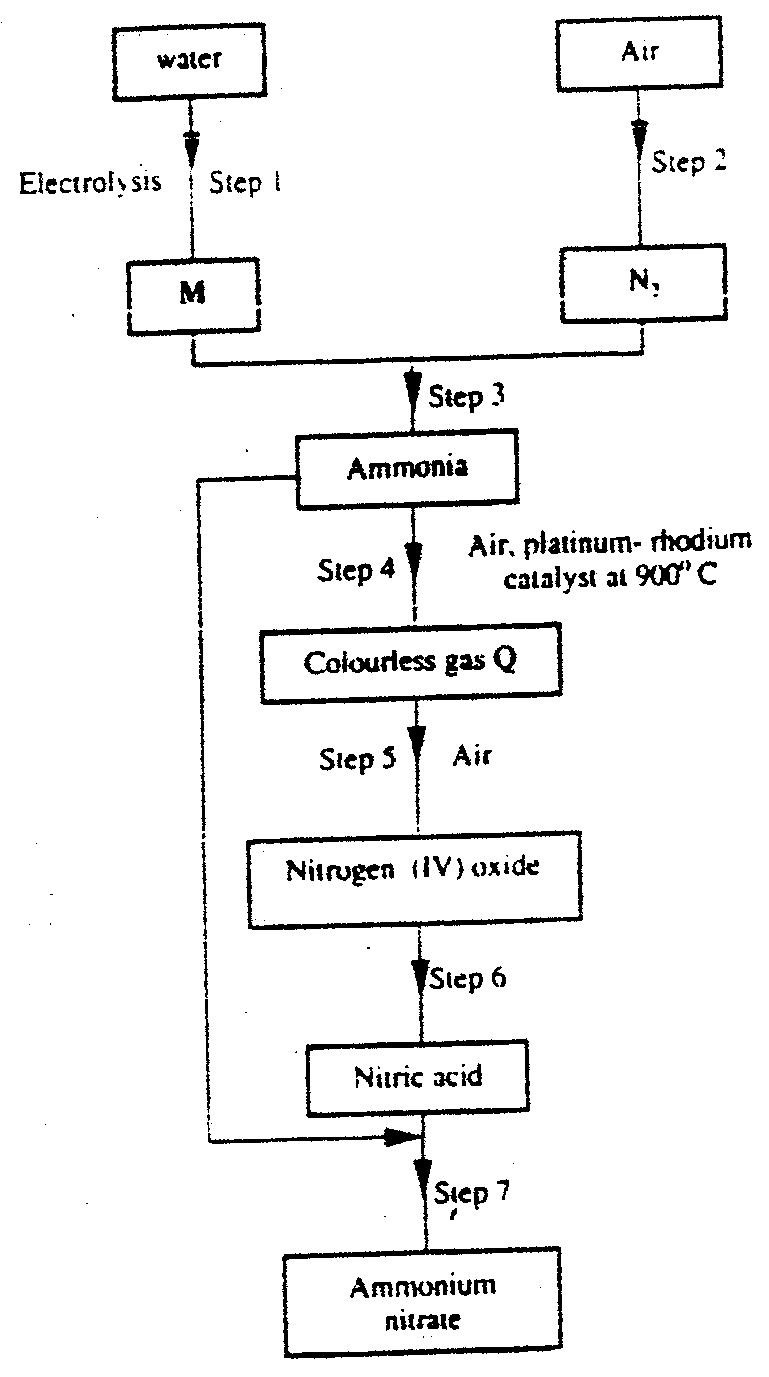
b) Study the flow chart below and answer the questions that follow.
The Ph of a sample of soil was found to be 5.0.An agricultural office recommended the addition of calcium oxide in the soil. State two functions of the calcium oxide in the soil.
Expected Response
Hydrogen peroxide decomposes according to the equation below:
8.5 gm of hydrogen peroxide contained in 100cm3 of solution with water were completely decomposed. Determine the rise in temperature due to the reaction. Specific density of water = 1g/cm3 O =16, H = 1,).
Expected Response
In an experiment to study the rate for reaction between duralumin (an alloy of aluminium, magnesium and copper) and hydrochloric acid, 0.5g of the alloy were reacted with excess 4M hydrochloric acid. The data in the table below was recoded.
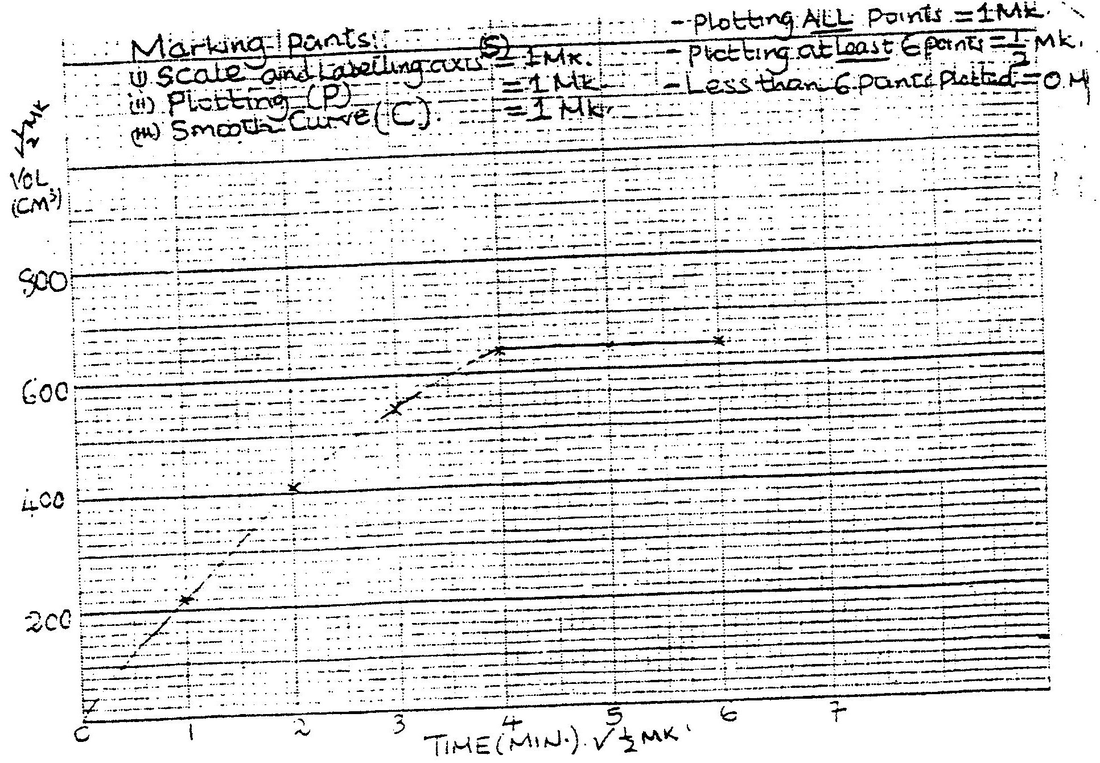
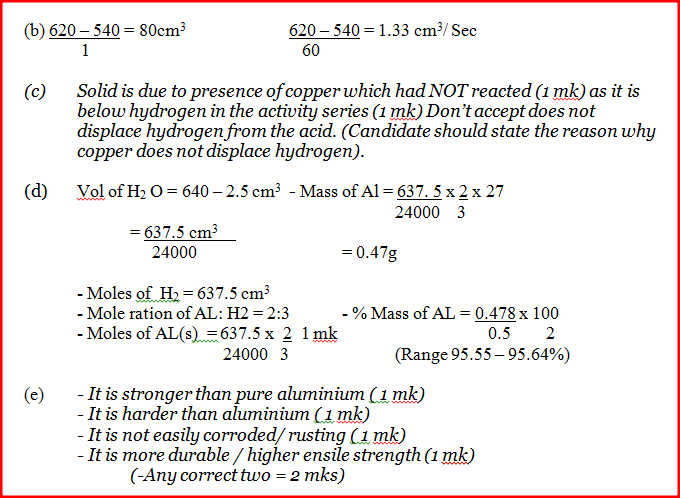
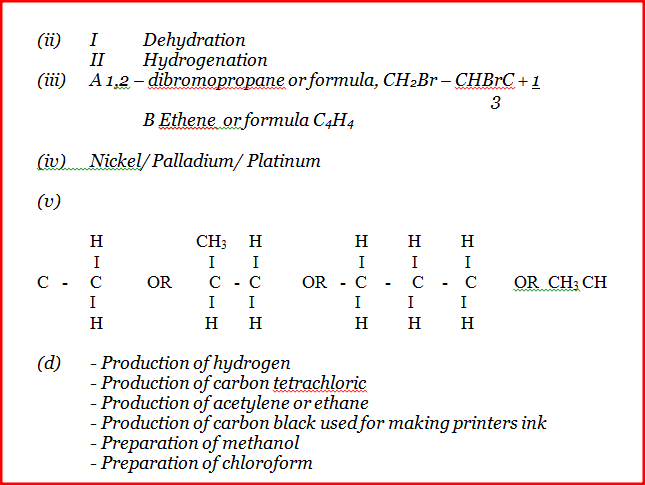
Use it to answer the questions that follow. a) i) On the grid provided, plot a graph of total volume of gas produced (vertical axis) again time. ii) From the graph, determine the volume of gas produced at the end of 2 ½ minutes. b) Determine the rate of reaction between the 3rd and 4th minute. c) Give a reason why some solid remained at the end of the experiment d) Given that 2.5cm3 of the total volume of the gas was from the reaction between magnesium and aqueous hydrochloric acid, calculate the percentage mass of aluminium present in 0.5g of the alloy. (Al = 27.0 and Molar gas volume = 24,000cm3 at 298k) e) State two properties of duralumin that make it more suitable than aluminium in aeroplane construction. a) In which homologous series do the following compounds belong i) CH3CC ii) CH3CH2COO
b) Raw rubber is heated with sulphur in the manufacture of natural rubber.
i) What is the name given to the process ii) Why is the process necessary?
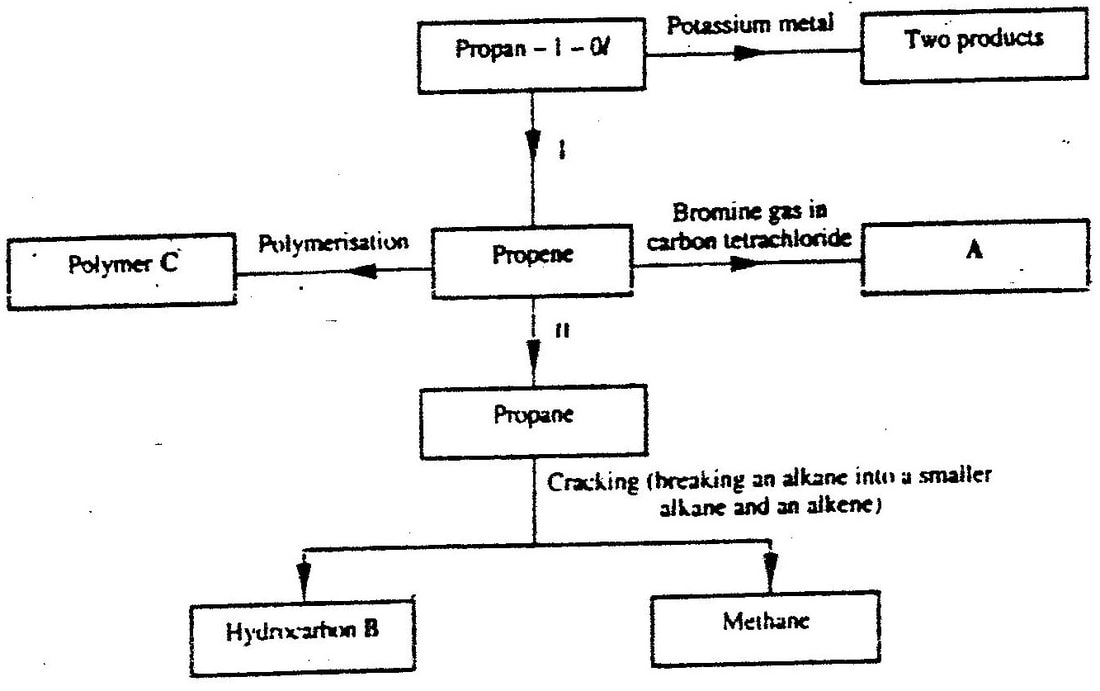
c) Study the scheme given below and answer the questions that follow
i) Write an equation for the reaction between propane – 1 – 0 / and potassium metal.
ii) Name processes I and II I II iii) Identify the products A and B iv) Name one catalyst used in process II v) Draw the structural formular of the repeating unit in the polymer C. d) State two industrial uses of methane.
a) Study the standard electrode potentials do the half – cells given below and
answer the questions that follow. (The letters do nor represent the actual symbols of the elements.)
i) Identify the strongest oxidizing agent. Give a reason for your answer.
ii) Which two half – cells would produce the highest potential difference when combined? iii) Explain whether the reaction represented below can take place. b) 100cm3 of 2M sulphuric acid was electrolysed using the set – up represented by the diagram below.
i) Write an equation for the reaction that produces gas L.
ii) Describe how gas K can be identified iii) Explain the difference in : I The volume of the gases produced at the electrodes. II Brightness of the bulb if 100cm3 of 2M ethanoic acid was used in place of sulphuric acid.
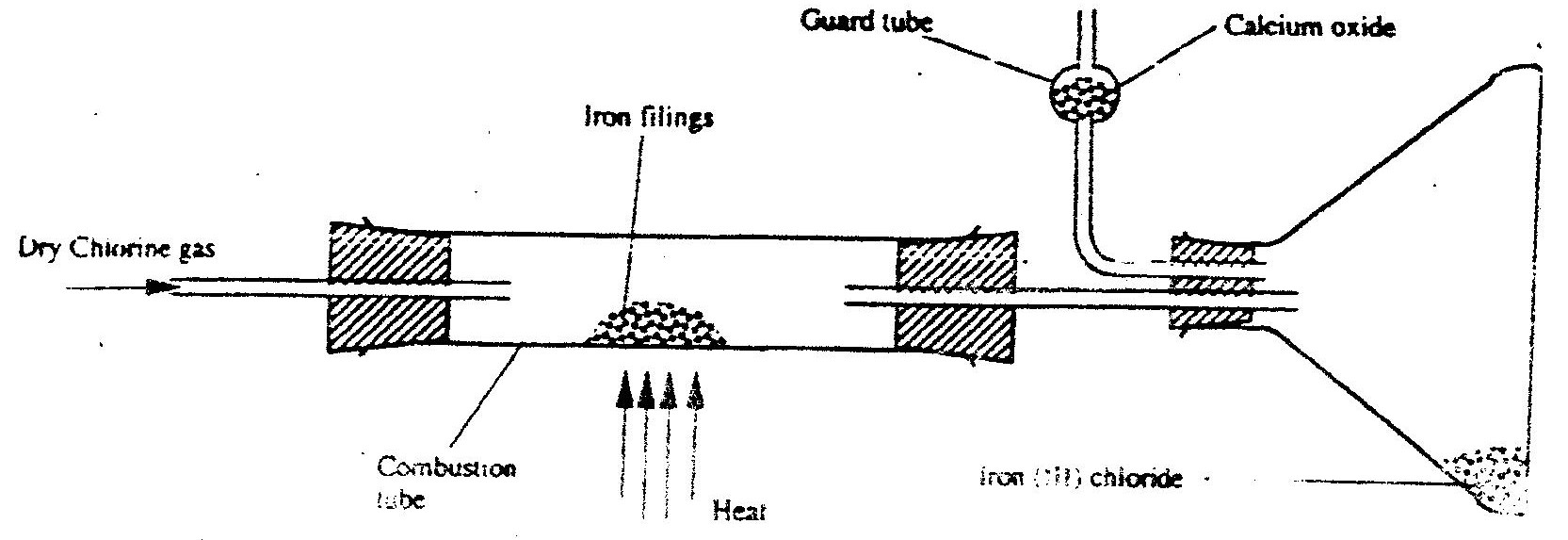
The apparatus shown below shown below was used to investigate the effect of carbon monoxide on copper (II)oxide
a) State the observation that was made in the combustion tube at the end of the experiment.
b) Write an equation for the reaction that took place in the combustion tube c) Why is it necessary to burn the gas coming out of tube K?
Explain why hydrogen forms compounds in which its oxidation state is either + 1 or -1 (Atomic number of hydrogen is 1)
The table below shows the properties of substances K,L,M and N
Select the substances which are likely to be:
a) Copper metal b) Magnesium chloride
Expected Response
(a) Copper metal M
(b) Magnesium chloride K
An element P has a relative atomic mass of 88. When a current of 0.5 amperes was passed through the fused chloride of P for 32 minutes and 10 seconds, 0.44g of P were deposited at the cathode. Determine the charge on an ion of P. (1 faraday = 96500 Coulombs).
Expected Response
The melting point of phosphorous dichloride is – 910C. While that of magnesium chloride is 7150C.In terms of structure and bonding, explain the difference in their melting points.
Expected Response
Explain why burning magnesium continues to burn a gas was bubbled
The burning magnesium produces more heat energy that the burning splint. The heat energy from magnesium is enough to break the sulphur oxygen bond setting free oxygen. Magnesium uses freed oxygen to continue burning.
Explain why a burning magnesium ribbon continues to burn when placed in a gas jar containing carbon (iv) oxide gas but a burning splint is extinguished.
(b) Write an equation for the reaction that takes place in (a) above.
a)what observation would be made if hydrogen sulphide gas was bubbled through a solution of zinc nitrate?
b) write an equation for the reaction that takes place in (a) above
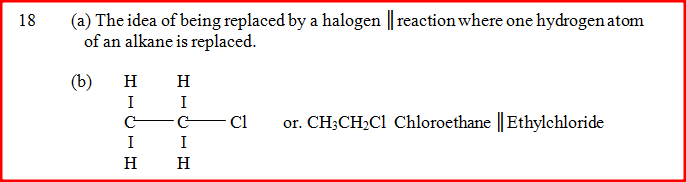
In the presence of U.V light, ethane gas undergoes substitution reaction with chlorine.
(a) What is meant by the term? Substitution reaction: (b) Give the structural formula and the name of the organic product formed when equal volumes of ethane and chlorine react together.
a) State one cause of temporary hardness in water.
b) How does distillation remove hardness from water?
Expected Response
(a) Presence of Ca (HCO3) or mg (HCO3)2 (b) Water vaporizes and distils off leaving behind ions that cause hardness
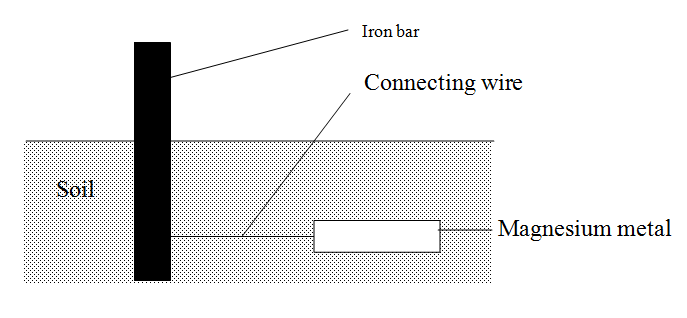
The diagram below shows an iron bar, which supports a bridge. The Iron bar is connected to a piece of magnesium metal
Explain why it is necessary to connect the piece of magnesium metal to the iron bar.
Expected Response
Magnesium is above iron in the activity series. It supplies electrons to the iron bar Hence prevent it from rusting
When a sample of concentrated sulphuric acid was left in an open beaker in a room for two days, the volume was found to have increased slightly
Expected Answer
(a) Hygroscopy
(b) Drying of gases ║drying agent
When the oxide of element H was heated with powdered carbon the mixture glowed and carbon dioxide was formed. When the experiment was repeated using the oxide of element J, there was no apparent reaction.
Expected Answer
(a) Electrolysis of fused or molten oxide
(b) JCH║J, carbon, H
The information in the table below relates to elements in the same group of the periodic table. Study it and answer the question that follows:
Which element has the highest ionization energy? Give reason.
Expected Answer
G3, because it has the smallest atomic radius. Its outer most electron is tightly held by the nucleus or it requires a lot of energy to remove it.
A certain matchstick head contains potassium chlorate and sulphure. On striking the two substances react to produce sulphure dioxide and potassium chloride. Explain the environmental effect of using such matches in large numbers.
Expected Answer
SO2 which is poisonous is released in the air. Acid rain which may cause corrosion will be formed
Describe a simple laboratory experiment that can be sued to distinguish between sodium and sulphide and sodium carbonate.
Expected Answer
Add dilute acid (e.g. HCI or H2SO4) to each substances separately. If Na2S, colourless gas, smell of rotten eggs 10gm of sodium hydrogen carbonate were dissolved in 20cm3 of water in a boiling tube. Lemon juice was then added drop wise with shaking until there was no further observable change.
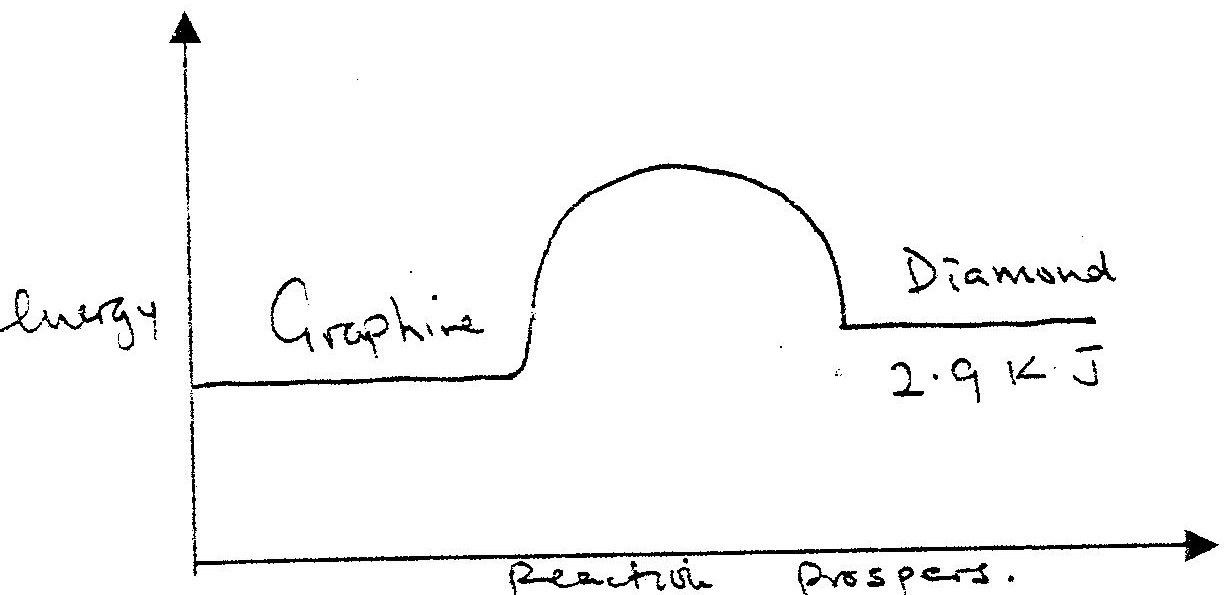
At 298K and 1 atmosphere, graphite changes into diamond according to the equation:
In the space provided, sketch a simple energy level diagram for the above change.
|
Chemistry Topics
All
Archives
December 2024
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
|
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |





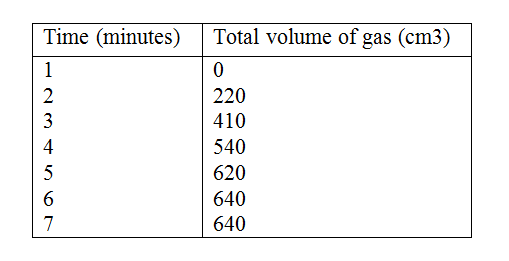


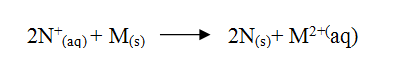














 RSS Feed
RSS Feed

