|
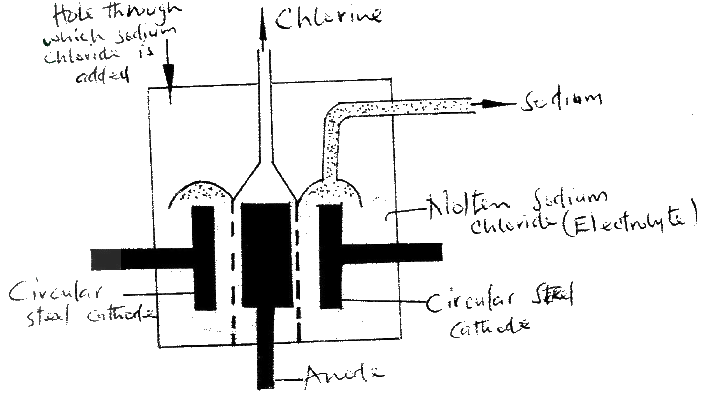
(a) Below is a simplified diagram of the Downs Cell used for the manufacture of sodium.
Study it and answer the questions that follow
(i) What material is the anode made of? Give a reason
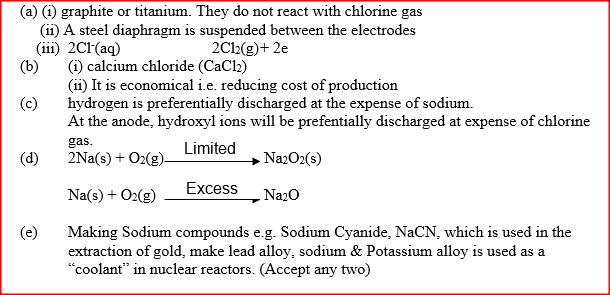
(ii) What precaution is taken to prevent chlorine and sodium from re- combination? (iii) Write an ionic equation for the reaction in which chlorine gas is formed (b) In the Downs process, (used for manufacture of sodium), a certain salt is added to lower the melting point of sodium chloride from about 800°C to about 600°C. (i) Name the salt that is added (ii) State why it is necessary to lower the temperature (c) Explain why aqueous sodium chloride is not suitable as an electrolyte for the manufacture of sodium in the Downs process (d) Sodium metal reacts with air to form two oxide. Give the formulae of two oxides
0 Comments
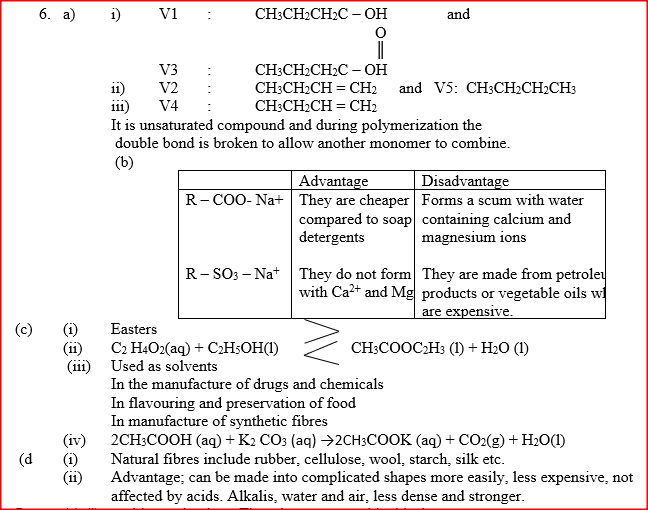
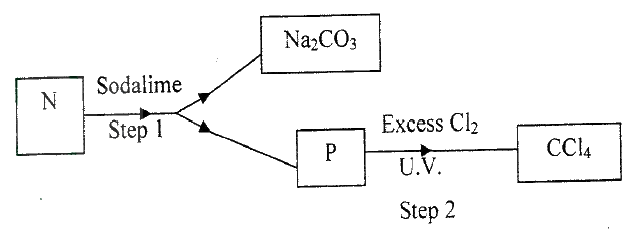
(a) The list below shows the formulae of some organic compounds. Use it to answer the questions that follow.
(i) Select two compounds whichI are not hydrocarbons II Belong to the same homologous series
(ii) Identify the compound that is likely to undergo polymerization. Give a reason for your answer. a. The structures below represents two cleansing agents: R – COO- Na+ R – OSO3- Na+ In the table below, give one advantage and one disadvantage of using each one of them
b. Under certain, ethanoic acid ( C2H4O2) and ethanol (C2H5OH) react to form a sweet smelling compound.
(i) What is the general name of compound to which the sweet smelling compound belong? (ii) Write the formula of the sweet smelling compound (iii) Give one use of ethanoic acid other the formation of the sweet smelling compounds (iv) Write the equation for the reaction dilute ethanoic acid and solid potassium carbonate c. Fibres are either synthetic or natural. Give one: (i) Example of a natural fibre (ii) Advantage of synthetic fibres have over natural fibres
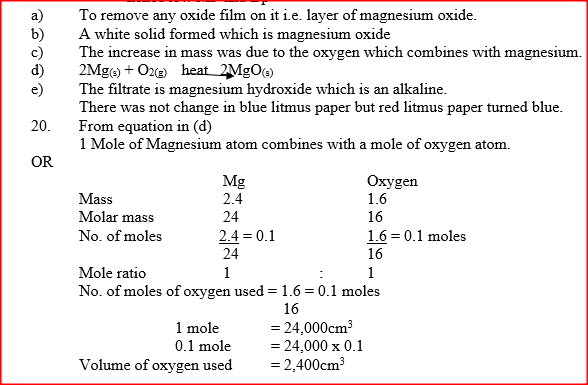
In an experiment, a piece of magnesium ribbon was cleaned with steel wool. 2.4 g of the clean magnesium ribbon was placed in a crucible and completely burnt in oxygen. After cooling, the product weighed 4.0 g
(a) Explain why it was necessary to clean the magnesium ribbon (b) What observation was made in the crucible after burning (c) Why was there an increase in mass? (d) Write the equation for the reaction which took place in the crucible (e) The product in the crucible was shaken with water and filtered. Explain the observation which was made when blue and red litmus papers were dropped into the filtrate.
(a) An atom Q can be represented as
What does the number 52 represent?
(b) Study the information in the table below and answer the equations that follow (Letters are not the actual symbols of the elements)
(i) Write the formula of the compound formed when N reacts with P. (atomic numbers are N = 20; P = 17)
(ii) Identify the elements which belong to the third period of the periodic table. Explain (iii) Which of the element identified in b (ii) above comes first in the third period? Explain (iv) Select two elements which are non- metals (c) The table below gives some properties of substances I, II, III, and IV. Study it and answer the questions that follow
(i) What type of bonding exists in substances I and II
I II (ii) Which substances is likely to be sulphur? Explain
(a) The table below shows the standard reduction potentials for four half- cells.
Study it and answer the questions that follow. (Letters are not the actual symbols of the elements)
(i) Identify the strongest reducing agent
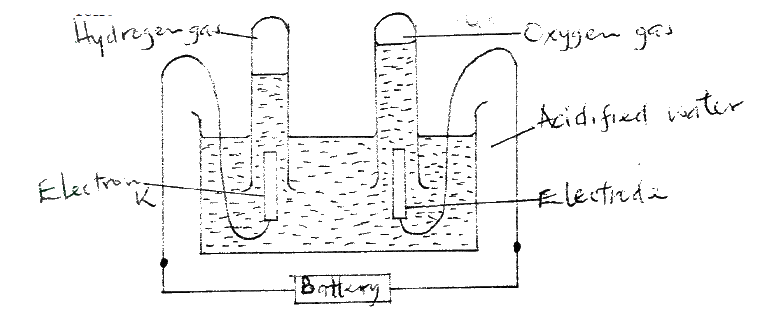
(ii) Write the equation for the reaction which takes place when solid G is added to a solution containing H2+ ions (iii) Calculate the Eθ value for the reaction in (ii) above (b) The diagram below shows the apparatus that can be used to electrolyze acidified water to obtain hydrogen and oxygen gases. Study it and answer the questions that follow
(i) Identify the electrode at which oxidation takes place
(ii) Give a reason why it is necessary to acidify the water (iii) Explain why hydrochloric acid is not used to acidify the water (c) During electrolysis of aqueous copper (II) sulphate, 144750 coulombs of electricity were used. Calculate the mass of copper metal that was obtained Cu = 64 ; 1 Faraday = 96500 coulombs)
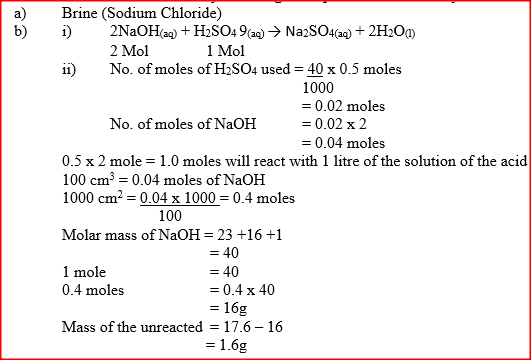
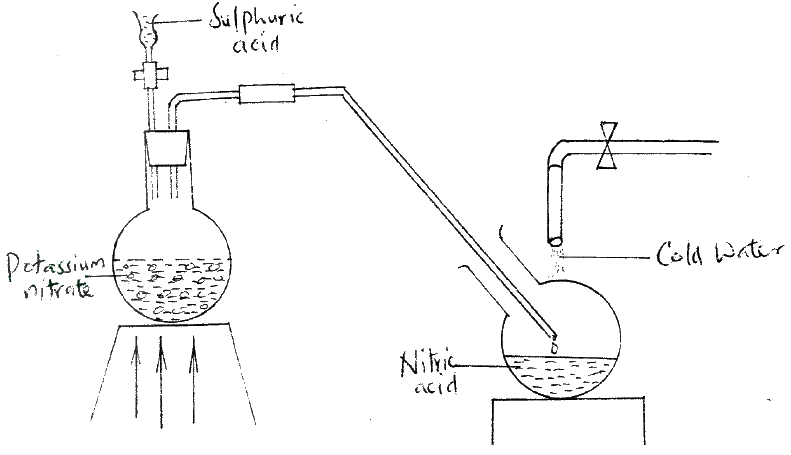
(a) Name one raw material which sodium hydroxide is manufactured
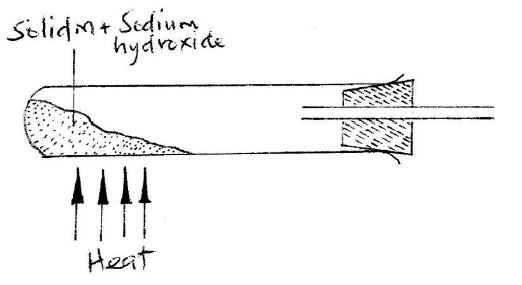
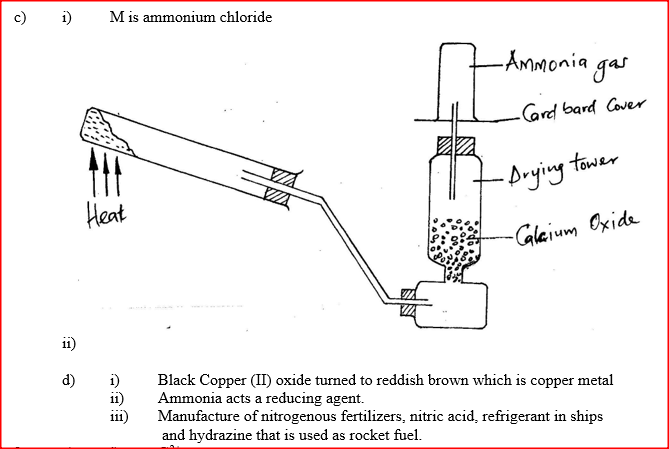
(b) Sodium hydroxide pellets were accidentally mixed with sodium chloride 17.6 g of the mixture were dissolved in water to make one litre of solution. 100 cm3 of the mixture were dissolved in water to make one litre solution. 100cm3 of the solution was neutralized by 40cm3 of 0.M sulphuric acid (i) Write an equation for the reaction that took place (ii) Calculate the: (i) Number of moles of the substance that reacted with sulphuric acid (ii) Number of moles of the substances that would react with sulphuric acid in the one litre of solution (iii) Mass of the unreacted substances in one litre of solution (H = 1,0 ; Na = 23.0 ; Cl= 35.5 ; 0= 16.0) (c) The diagram below shows an incomplete set-up used to prepare and collect ammonia gas
(i) Name solid M
(ii) Complete the diagram to show how a dry sample of ammonia gas can be collected (d) In an experiment, excess ammonia gas passed over heated copper (II) oxide on a combustion tube. (i) State the observation that was made in the combustion tube at the end of the experiment (ii) What property of ammonia is shown in the above reaction (iii) Name one use of ammonia
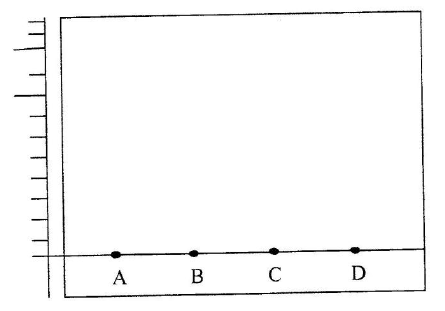
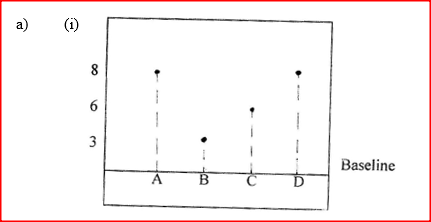
(a) The diagram below shows spots of pure substance A,B, and C on a chromatography paper. Spot D is that of a mixture
After development, A, B and C were found to have moved 8cm, 3cm and 6 cm respectively. D has separated into two spots which had moved 6cm and 8 cm
(i) On the diagram I Label the baseline ( origin) II Show the positions of all the spots after development (ii) Identify the substances present in the mixture D (b) Describe how solid ammonium chloride can be separated fro a solid mixture of ammonium chloride and anhydrous calcium chloride (c) The table shows liquids that are miscible and those that are immiscible
Use the information given to answer the questions that follow
(i) Name the method that can be used to separate L1 and L3 from a mixture of two (ii) Describe how a mixture of L2 and L4 can be separated
ANSWERS
(ii) A and C b)Since NH4CL4 sublimes but CaCl2 does not; sublimation process would do. Heat the mixture. Ammonium chloride sublimates into vapour and condenses on the cooler part of the heating tube. Calcium chloride will remain on the bottom of the heating tube. c)i)Fractional distillation ii)Separating funnel method Since the tow liquids are immiscible, pour both the liquids in a separating funnel and allow to settle, the denser liquid will settle down and the less dense will form a second layer on top. Open the tape and run out the liquid in the bottom layer leaving the liquid in the second layer in the funnel.
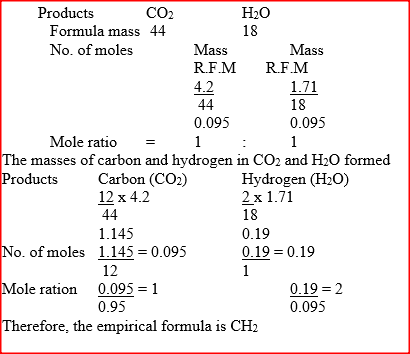
When a hydrocarbon was completely burnt in oxygen, 4.2g of carbon dioxide and 1.71 g of water were formed. Determine the empirical formula of the hydrocarbon
(H= 1.0 ; C=12.0 ; 0 = 16.0)
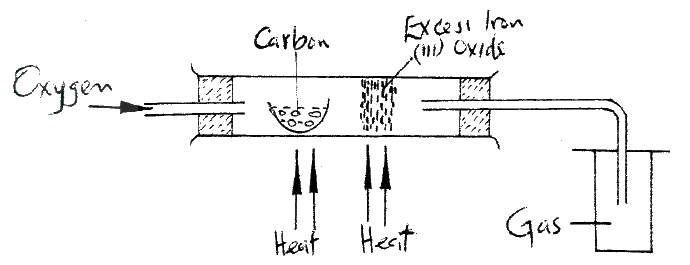
Dry carbon monoxide gas reacts with heated lead (II) oxide as shown in the equation below
PbO(s) + CO(g) →Pb (s) + CO2 (g) (a) Name the process undergone by the lead (II) oxide (b) Give a reason for your answer in (a) above (c) Name another gas that can be used to perform the same function as carbon monoxide gas in the above reaction.
ANSWERS
When a current of 0.82A was passed for 5 hours through an aqueous solution of metal Z, 2.65 g of the metal were deposited. Determine the charge on the ions of metal Z. ( 1 Faraday = 96500 Coulombs:
Relative atomic mass of Z = 52
When a few drops of aqueous ammonia were added to copper (II) nitrate solution, a light tube precipitate was formed. On addition of more aqueous ammonia, a deep blue solution was formed.
Identify the substance responsible for the: a. Light blue precipitate b. Deep blue solution
ANSWERS
In an experiment, a gas jar containing most sulphur dioxide was inverted over another gas jar containing hydrogen sulphide gas
a. State and explain the observation that was made b. State the precaution that should be taken when carrying out this experiment
ANSWERS
ANSWERS
ANSWERS
ANSWERS
ANSWERS
(a) what condition is necessary for an equilibrium to be established?
(b) When calcium carbonate is heated, the equilibrium shown below is established CaCO3 (s) → CaO(s) + CO2(g) How would the position of equilibrium be affected if a small amount of dilute potassium hydroxide is added to the equilibrium mixture? Explain
ANSWERS
ANSWERS
In terms of structure and bonding, explain why graphite is used as a lubricant.
ANSWERS
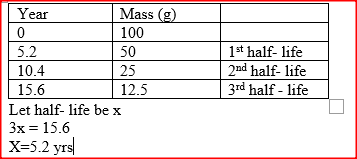
100 g of a radioactive substance was reduced to 12.5 g in 15.6 years. Calculate the half – life of the substance
Methane reacts with oxygen according to the equation given below.
CH4 (g) + 2O2 (g) →CO2 (g) + 2H2O (l), ΔH = 890 KJ MOL-1 Calculate the volume of methane which would produce 111.25 kj when completely burnt.(Molar volume of a gas = 24 litres.)
In the industrial extraction of lead, the ore is first roasted in a furnace. The solid mixture obtained is then fed into another furnace together with coke, limestone and scarp iron. State the function of each of the following in this process:
(a) Coke (b) Limestone (c) Scrap iron
The reaction between how concentrated sodium hydroxide and chlorine produces sodium chlorate (V), sodium chloride and water
(a) Write the equation for the reaction (b) Give one use of sodium chlorate (V)
ANSWERS
|
Chemistry Topics
All
Archives
December 2024
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
|
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |
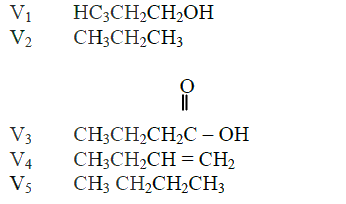
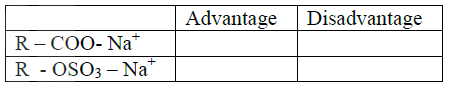
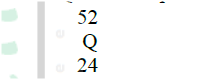

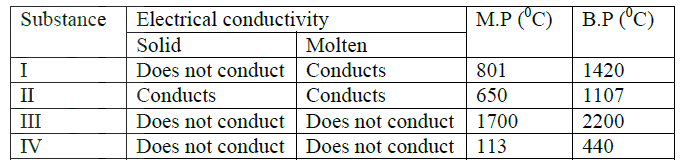

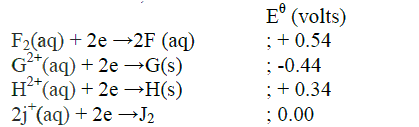
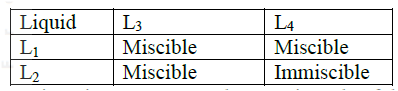
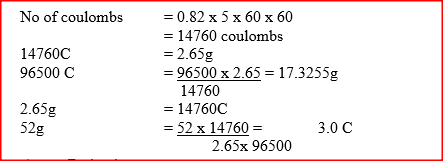
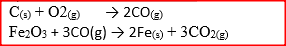
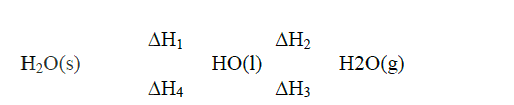
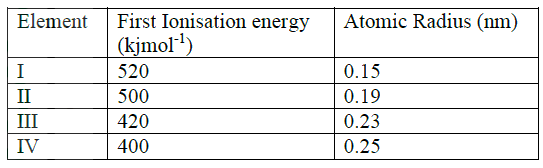





 RSS Feed
RSS Feed

