|
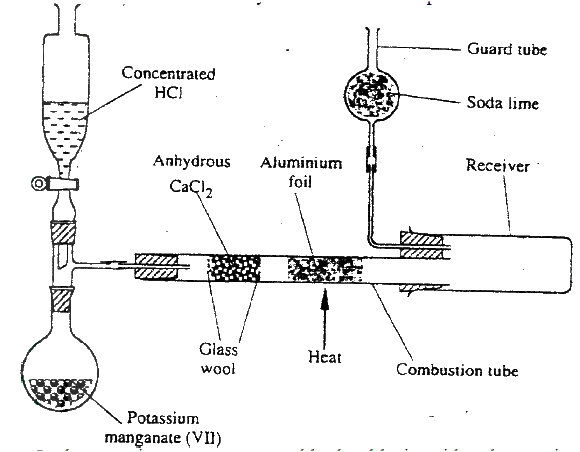
The diagram below shows the set up used in an experiment to prepare chlorine gas and react it with aluminium foil. Study it and answer the question that follow
(a) In the experiment, concentrated hydrochloric acid and potassium manganate (VII) were used to prepare chlorine gas. State two precautions that should be taken in carrying out this experiment.
(b) Write the formula of another compound that could be used instead of potassium manganate (VII) (c) Explain why it is necessary to allow the acid to drip slowly onto potassium manganate (VII) before the aluminium foil is heated. (d) State the property of the product formed in the combustion tube that makes it possible for it to be collected in the receiver (e) When 1.08g of aluminum foil were heated in a stream of chlorine gas, the mass of the product formed was 3.47 g Calculate the: (i) Maximum mass of the product formed if chlorine was in excess; (Al= 27; Cl = 35.5) (ii) Percentage yield of the product formed (f) Phosphorous trichloride is a liquid at room temperature. What modification should be made to set up if it is to be used to prepare phosphorous trichloride?
0 Comments
(a) The elements nitrogen, phosphorous and potassium are essential for plant growth.
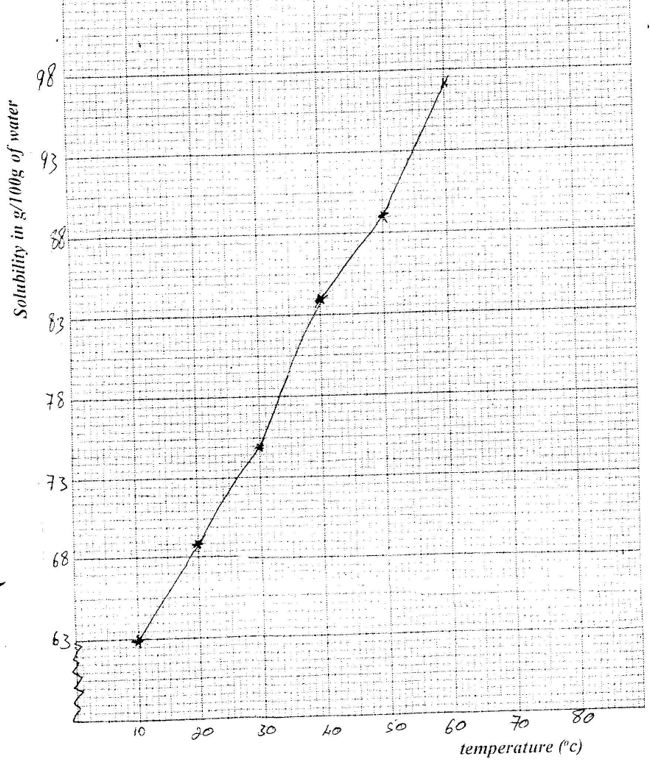
(i) Potassium in fertilizers may be in the form of potassium nitrate Describe how a sample of a fertilizer may be tested to find out if it contained nitrate ions. (ii) Calculate the mass of nitrogen present if a 25kg bag contained pure ammonium phosphate, (NH4)2 HPO4. (N = 14.0, H=1.0, P = 31.0, O = 16.0 (b) The table below shows the solubility of ammonium phosphate in water at different temperatures.
(i) On the grid provided, draw the solubility curve of ammonium phosphate (Temperature on x – axis)
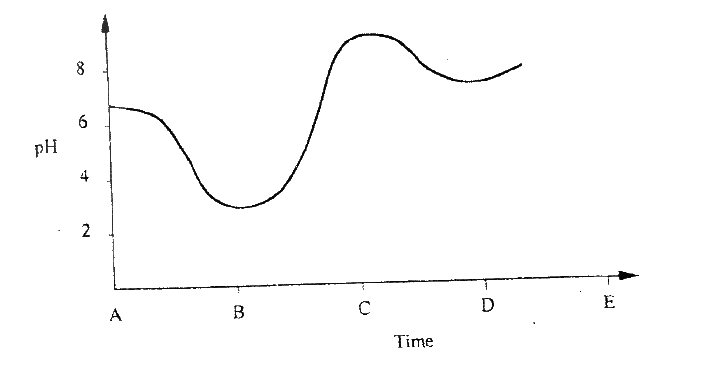
(ii) Using the graph, determine the solubility of ammonium phosphate at 25°C (iii) 100g of a saturated solution of ammonium phosphate was prepared at 25°C I what is meant by a saturated solution? II Calculate the mass of ammonium phosphate which was used to prepare the saturated solution (c) The graph below shows how the PH value of soil in a farm changed over a period of time
(i) Describe how the pH of the soil can be determined
(ii) State one factor that may have been responsible for the change in the soil pH in the time interval AB
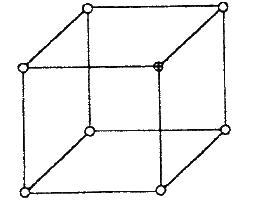
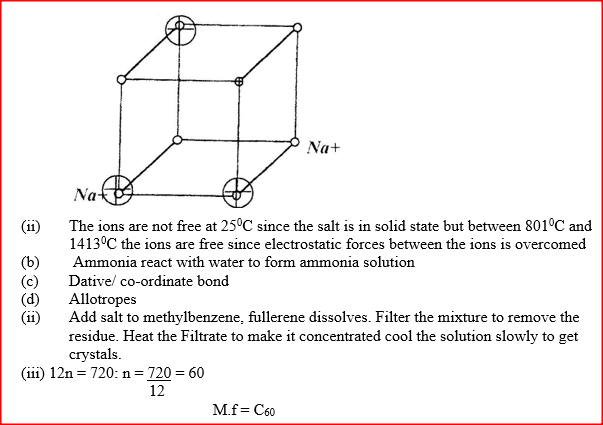
(a) The diagram below represents part of the structure of a sodium chloride crystal.
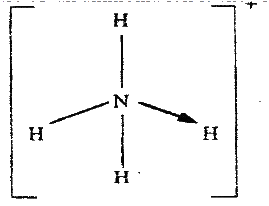
The position of one of the sodium ions in the crystal is shown as (i) On the diagram, mark the position of the other three sodium ions (ii) The melting and boiling points of sodium chloride are 801°C and 1413°C respectively. Explain why sodium chloride does not conduct electricity at 25°C, but does so at temperatures between 801° C and 1413°C (b) Give a reason why ammonia gas is highly soluble in water (c) The structure of an ammonia ion is shown below:
Name the type of bond represented in the diagram by N → H
(d) Carbon exists in different crystalline forms. Some of these forms were recently discovered in soot and are called fullerenes (i) What name is given to different crystalline forms of the same element? (ii) Fullerenes dissolve in methylbenzene while the other forms of carbon do not. Given that soot is a mixture of fullerenes and other solid forms of carbon, describe how crystals of fullerenes can be obtained from soot. (iii) The relative molecular mass of one of the fullerenes is 720. What is the molecular formula of this fullerene? (C=12.0)
(a) Methanol is manufactured from carbon (IV) oxide and hydrogen gas according to the equation:
The reaction is carried out in the presence of a chromium catalyst at 700K and 30kPa. Under these conditions, equilibrium is reacted when 2% of the carbon (IV) oxide is converted to methanol
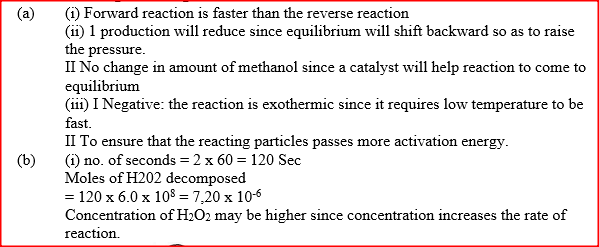
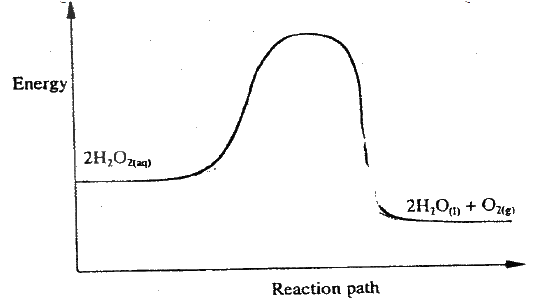
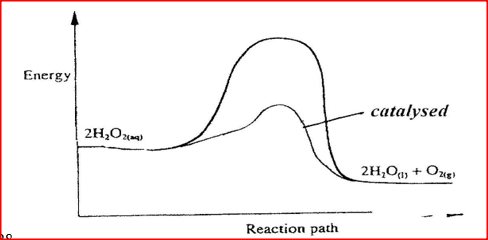
(i) How does the rate of the forward reaction compare with that of the reverse reaction when 2% of the carbon (IV) oxide is converted to methanol? (ii) Explain how each of the following would affect the yield of methanol: I Reduction II Using a more efficient catalyst (iii) If the reaction is carried out at 500K and 30kPa, the percentage of carbon (IV) oxide converted to methanol is higher than 2% I what is the sign of ΔH for the reaction? Give a reason II Explain why in practice the reaction is carried out at 700K but NOT at 500K (b) Hydrogen peroxide decomposes according to the following equation : 2H2O2(aq) →2H2O(l) + O2 (g) In an experiment, the rate of decomposition of hydrogen peroxide was found to be 6.0 x 10-8 mol dm-3 S-1. (i) Calculate the number of moles per dm3 of hydrogen peroxide that had decomposed within the first 2 minutes (ii) In another experiment, the rate of decomposition was found to be 1.8 x 10-7 mol dm-3S-1. The difference in two rates could have been caused by addition of a catalyst. State, giving reasons, one other factor that may have caused the difference in two rates of decomposition
The flow chart below shows a sequence of chemical reactions starting with copper study it and answer the questions that follow.
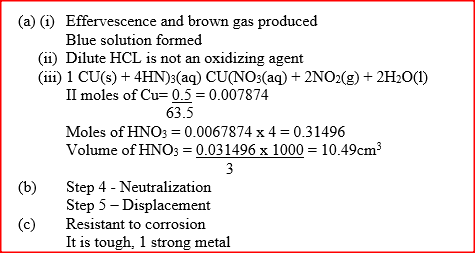
(a) In step 1, excess 3M nitric acid was added to 0.5g of copper powder
(i) State two observations which were made when the reactions was in progress (ii) Explain why dilute hydrochloric acid cannot be used in step 1 (iii) I Write the equation for the reaction that took place in step 1 II Calculate the volume of 3M nitric that was needed to react completely with 0.5g of copper powder. (Cu = 63.5) (b) Give the names of the types of reactions that took place in steps 4 and 5 Step 4 Step 5 (c) Apart from the good conductivity of electricity, state two other properties that make it possible for copper to be extensively used in the electrical industry.
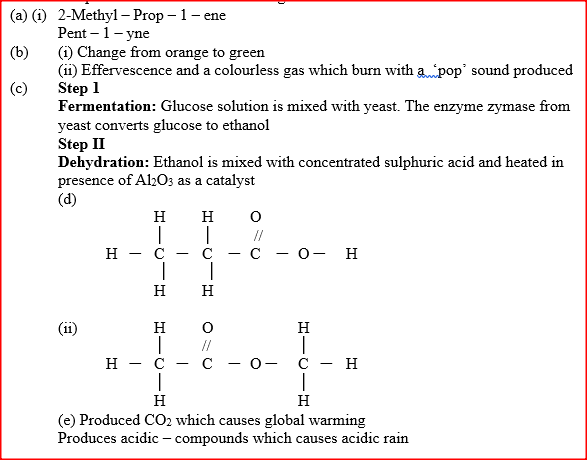
(a) Give the systematic names of the following compounds
(b) State the observations made when Propan – 1- ol reacts with:
(i) Acidified potassium dichromate (VI) Solution (ii) Sodium metal (c) Ethanol obtained from glucose can be converted to ethane as shown below
Name and describe the process that take place in steps I and IIStep I Step II
(d) Compounds A and B have the same molecular formula C3H6O2. Compound A liberates carbon (IV) oxide on addition of aqueous sodium carbonate while compound B does not. Compound B has a sweet smell. Draw the possible structures of: (i) Compound A (ii) Compound B (e) Give two reasons why the disposal of polymers such as polychloroethane by burning pollutes the environment.
(a) State two factors that should be considered when choosing fuel for cooking
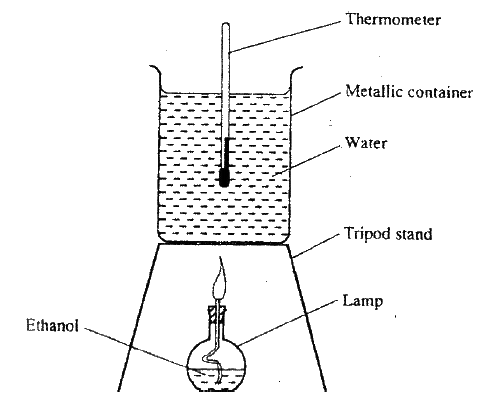
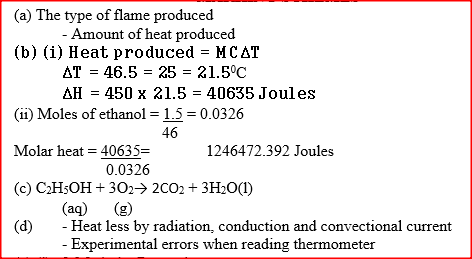
(b) The diagram below represents a set – up that was used to determine the molar heat of combustion of ethanol
During the experiment, the data given below was recorded
Calculate the:
(i) Heat evolved during the experiment (density of water = 1g/cm3 Specific heat capacity of water = 4.2 Jg-1K-1 (ii) Molar heat of combustion of ethanol (C = 12.0, O = 16.0, H=1.0) (c) Write the equation for the complete combustion of ethanol (d) The value of the molar heat of combustion of ethanol obtained in (b) (ii) above is lower than the theoretical value. State two sources of error in the experiment.
ANSWER
ANSWERS
(a) Metallic bonding
(b) Group 1 Each atom contains one electron in its outer most energy level
During the electrolysis of aqueous silver nitrate, a current of 5.0a was passed through the electrolysis for 3 hours.
a) Write the equation for reaction which took place at the anode. b) Calculate the mass of silver deposited (Ag = 108; IF=96500C)
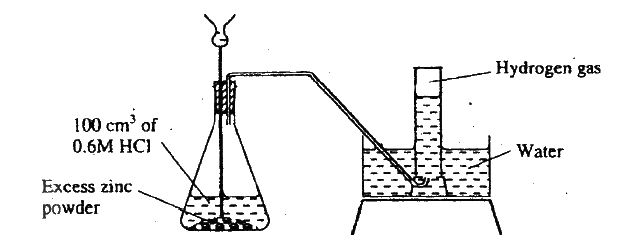
The diagram below shows a student's set-up for the preparation and collection of hydrogen gas.
(a) How would the final volume of hydrogen gas produced be affected if 80cm3 of 0,75M hydrochloric acid was used?
(b) Give a reason why helium is increasingly being preferred to hydrogen in weather balloons.
ANSWERS
(a) No change in volume since the number of moles of acid is equal in both cases.
(b) It is less dense and does not burn like hydrogen
State and explain the observations made when excess ammonia gas reacts with chlorine gas
ANSWERS
ANSWERS
6.84g of aluminium sulphate were dissolve in 150cm3 of water. Calculate the molar concentration of the sulphate ions in the solution. (Relative formula mass of aluminium sulphate is 342)
a) When brine is electrolyzed using inert electrodes, chlorine gas is liberated at the anode instead of oxygen. Explain this observation.
b) Name the product formed at the cathode.
ANSWERS
(a)Chlorine ions in Brine are high concentration compared to oxide ions in solutions
(b)Hydrogen gas
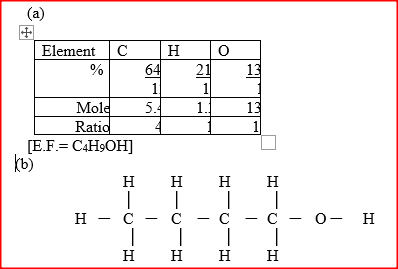
An alcohol has the following composition by mass: hydrogen 13.5%, oxygen 21.6% and carbon 64.9%
a) Determine the empirical formula of the alcohol(C=12.0; H=1.0)=16.0). b) Draw the structural formulae of the compound,
Starting with sodium metal, describe how a sample of crystals of sodium hydrogen carbonate may be prepared.
ANSWERS
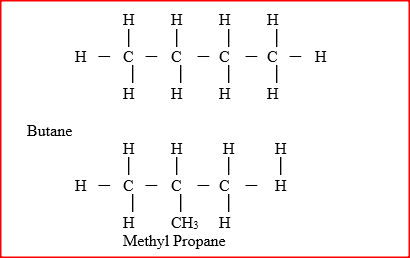
The relative formula mass of a hydrocarbon is 58. Draw and name two possible structures of the hydrocarbon (C=12.0; H=1.0)
a) Explain why permanent hardness in water cannot be removed by boiling.
b) Name two methods that can be used to remove permanent hardness from water.
ANSWERS
(a) The calcium and magnesium compounds in this water can not be decomposed by heating i.e. CaCl2, CaSO4, MgSO4 and MgCl2
(b)Ionic exchange Uses sodium carbonate (washing soda)
a) Distinguish between nuclear fission and nuclear fusion.
b) Describe how solid wastes containing radioactive substances should be disposed of.
ANSWERS
(a) Nuclear fusion is where two light nuclei combine to give a heavy release of energy while nuclear fusion is where a large nuclear splits into smaller nuclei with the release of enormous amount of energy.
(b) Wrap with aluminium or lead foil and bury them deep underground
a) State the Charles law
b) The volume of a sample of nitrogen gas at a temperature of 291 K and 1.0x105 Pascal‟s was 3.5 x 10-2m3. Calculate the temperature at which the volume of the gas would be 2.8 x 10-2m3 at 1.0 x 105 Pascal. |
Chemistry Topics
All
Archives
December 2024
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
|
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |



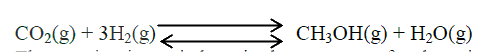
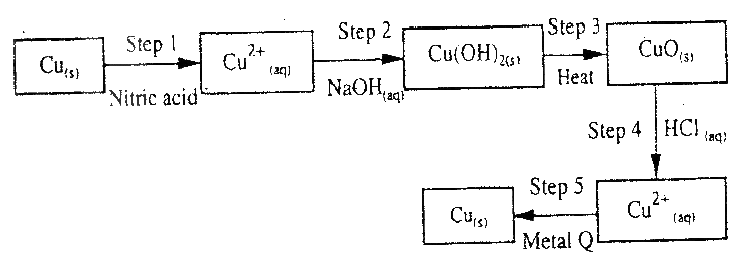
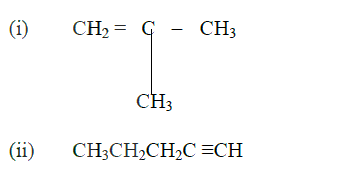
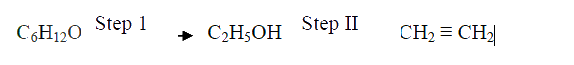
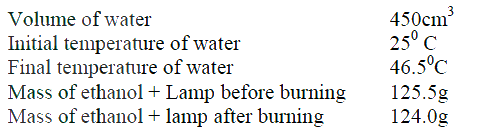
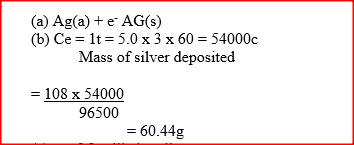

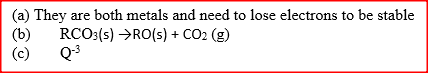
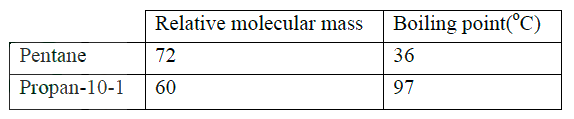
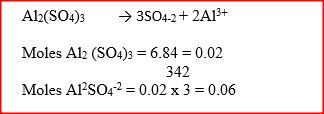




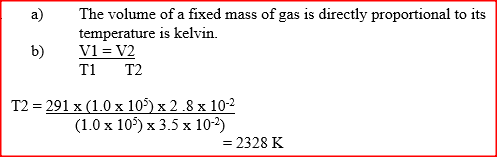

 RSS Feed
RSS Feed

