|
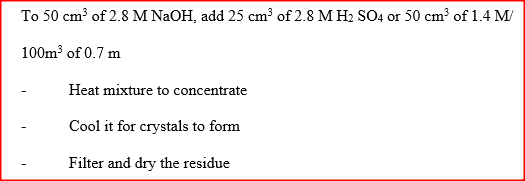
Starting with 50 cm3 of 2.8M sodium hydroxide, describe how a sample of pure sodium sulphate crystals can be prepared.
0 Comments
Hydrogen and oxygen can be obtained by electrolysis of acidified water.
Using equations for the reactions at the electrodes, explain why the volume of hydrogen obtained is twice that of oxygen. Related Chemistry Questions and Answers on Electrochemistry Form 4 Level
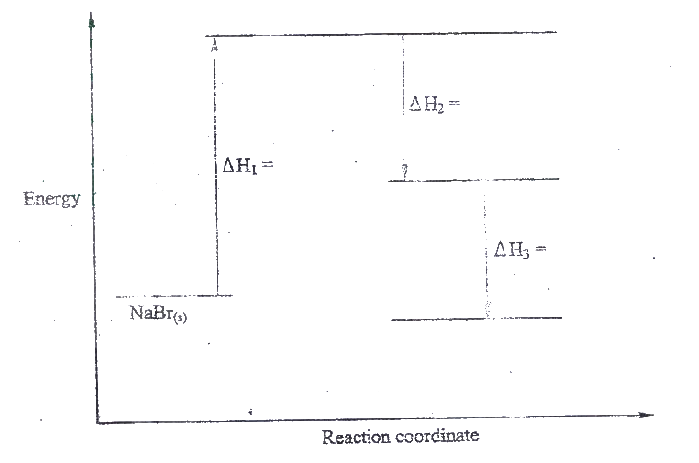
a) what is meant by molar heat of solution?
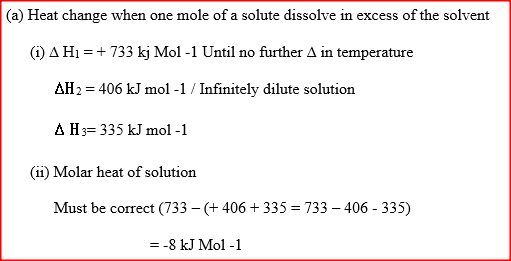
b) the lattice energy of sodium bromide and hydration energies of sodium and bromide ions are: 733,406 and 335 kJmol -1 respectively. i) Complete the energy cycle diagram below by inserting the values of ΔH1, ΔH2, and ΔH3
ii) Determine the molar heat of solution of solid sodium bromide.
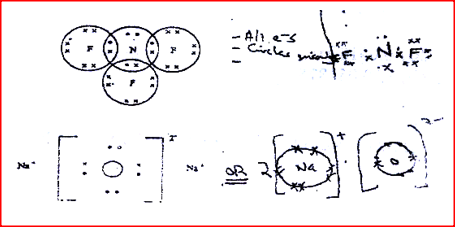
Using dots (.) and crosses (x) , show bonding in:
a) The compound formed when nitrogen reacts with fluorine (Atomic numbers F=9, N=7); b) Sodium oxide.(Atomic numbers Na= 11, 0 = 8)
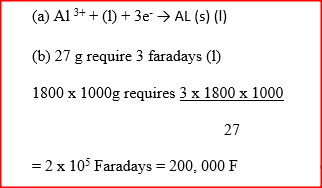
When aluminium oxide was electrolysed, 1800kg of aluminium metal were obtained.
a) Write equation for the formation of aluminium metal b) Calculate the quantity of electricity in faradays used (Al=27)
An isotope of element E has 34 neutrons and its mass number is 64. E forms a cation with 28 electrons. Write the formula of the cation with 28 electrons. Write the formula of the cation indicating the mass and atomic numbers.
In terms of structure and bonding, explain why the melting point of oxygen is much lower than that of sodium.
ANSWER
ANSWERS
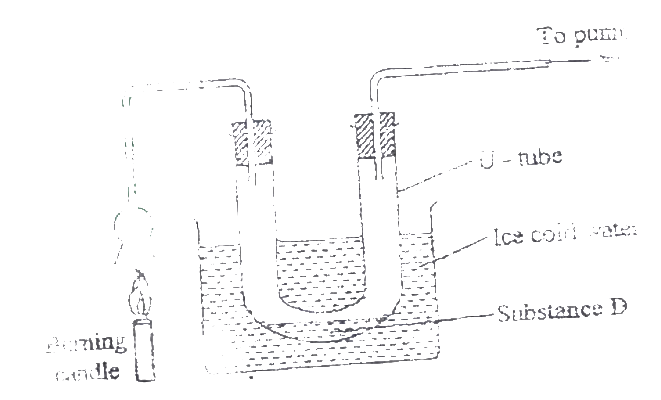
(a) Water
(b) The second / other product of burning candle is carbon (IV) oxide (l). It can be prevented from getting into the environment by passing it though a hydroxide solution/ alkaline solution e.g. K.O.H NaOH or aqueous ammonia . To form K2CO3
ANSWERS
(i) 2.8.8 (ii) 2.8.2
Hardness of water may be removed by either boiling or addition of chemicals
a) write an equation to show how boiling removes hardness of water. b) name two chemicals that are used to remove hardness of water.
ANSWERS
(a) Energy required to remove 1 mole of electrons from 1 mole of gaseous atoms
(b) B 418??? It loses electrons most readily Reject lowest i.e. Mg (HCO3) 2 aq →MgCS O3 + H2O + CO2 (g) |
Chemistry Topics
All
Archives
December 2024
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
|
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |
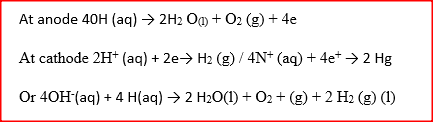


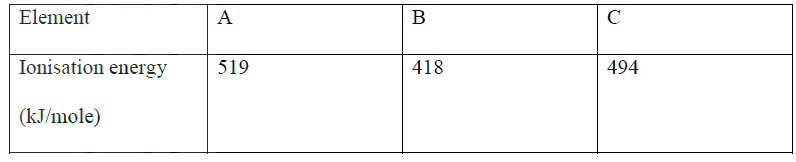

 RSS Feed
RSS Feed

