|
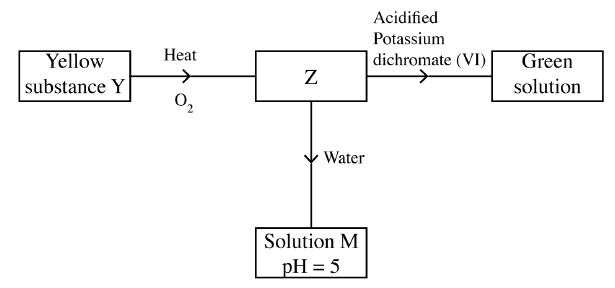
Study the flow chart below and answer the questions t hat follow.
Identify Z and M.
Z……………………… M……………………
0 Comments
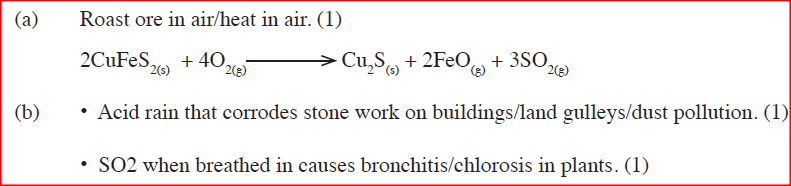
One of the ores of copper has formula, CuFeS2.
(a) Describe how iron in the ore is removed during concentration of copper metal. (b) State two environmental problems associated with extraction of copper metal.
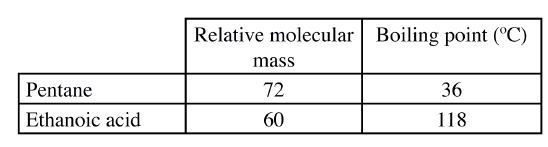
The table below shows the relative molecular masses and boiling points of pentane and ethanoic acid
Explain the large difference in boiling point between ethanoic acid and pentane.
ANSWERS
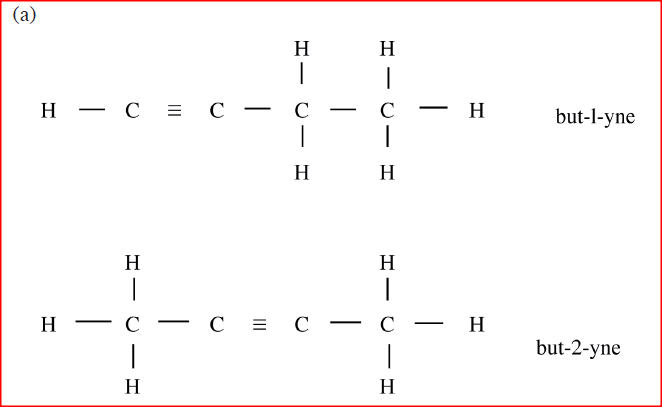
(a) Draw and name the isomers of butyne
(b) State one use of polystyrene.
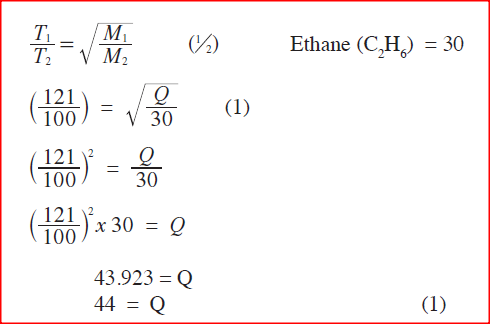
100cm3 of a sample of ethane gas diffuses through a porous pot in 100 seconds. What is the molecular mass of gas Q if 1000 cm3 of the gas diffuses through the same porous not in 121 seconds under the same conditions? (C=12.0, H=1.0)
Explain how condition of electricity takes place in the following.
(a) Iron metal (b) Molten lead (II) iodide
ANSWERS
(a) delocalised electrons.
(b) Ions in the melt.
Starting with zinc sulphate solution, describe how a sample of zinc oxide can be obtained
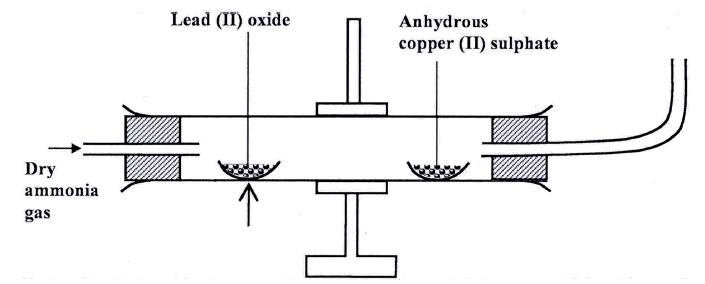
Dry ammonia gas was passed over heated lead (II) oxide and the product passed over anhydrous Copper (II) sulphate as shown in the diagram below.
State:
(a)Two observations made in the combustion tube. (b) The property of ammonia gas shown in this experiment
ANSWERS
(a)The anhydrous copper (II) Sulphate turns from white to blue.
A grey solid is formed/droplets of a colourless liquid condense at cool part (b)Reducing property.
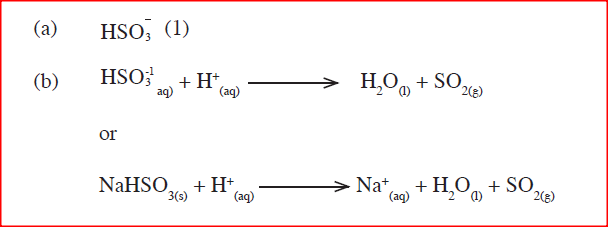
When dilute hydrochloric acid was reacted with solid B, a colourless gas which extinguished a burning splint was produced. When an aqueous solution of solid B was tested with a blue litmus paper, the paper turned red / pink.
(a) Identify the anion present in solid B. (b) Write an ionic equation for the reaction between solid B and dilute hydrochloric acid.
Explain how the hotness of a Bunsen burner flame can be increased
ANSWER
|
Chemistry Topics
All
Archives
December 2024
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
|
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |




 RSS Feed
RSS Feed

