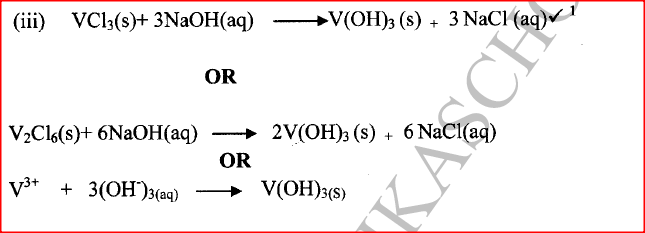
|
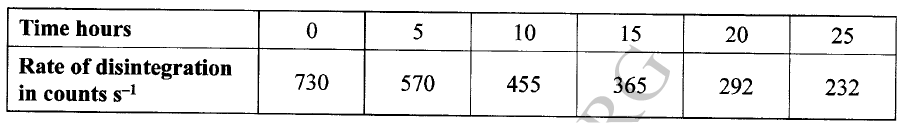
The decay rates of a sample of a radioisotope of bismuth at different time intervals is indicated in the following table.
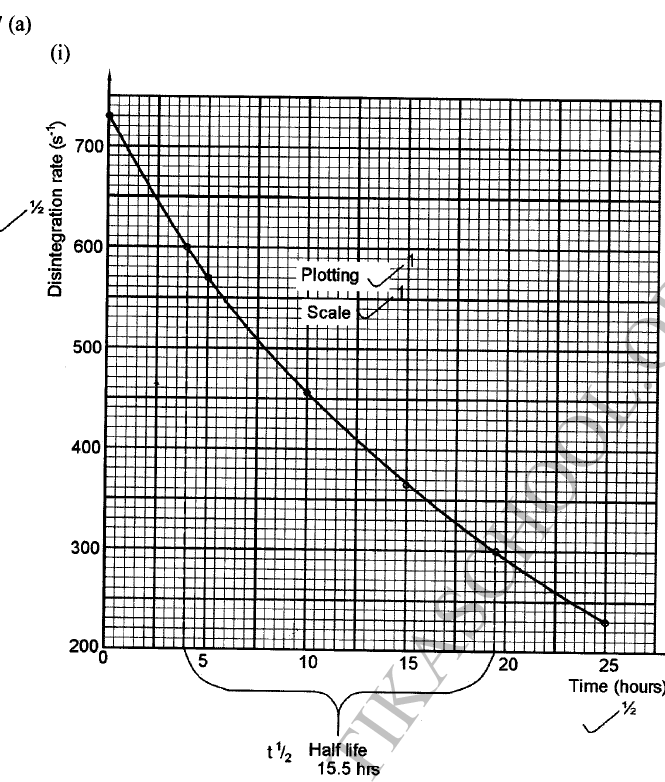
(a)(i) Draw a graph of disintegration rate against time.
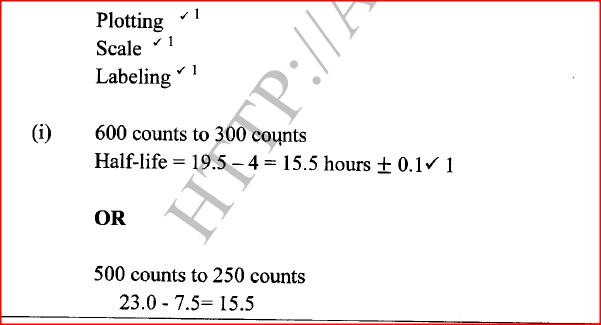
(ii) Determine the half-life of bismuth. (iii) What would be the effect on the curve if half the amount of sample of bismuth were used. (b) Radioactivity has several applications. State one application of radioactivity in: (i) Medicine (ii) Agriculture (iii) Tracers (iv) Nuclear power station (c) State two dangers associated with radioactivity.
ANSWERS
(ii) It would have no effect on the curve as the quantity of bismuth does not affect half-life.
(b)(i)Applications in medicine Sterilizing surgical instruments. Destroying cancerous tissues during radiotherapy. Provide power to the heart pace setters. (ii) Applications in agriculture Monitor photosynthesis and other related processes. Preservation of foodstuffs, by exposing Micro-organisms to gamma rays. Rate of absorption of a fertilizer by the plant. (iii)Applications in Tracers Detecting leakages in underground water or oil pipes. (iv)Applications in Nuclear power stations. To generate electricity. (d) Dangers of radioactivity Long term exposure causes genetic mutation; Radioisotopes can be used as weapon of mass Destruction; Causes skin cancer; When tested causes environmental pollution.
0 Comments
The following steps were used to analyse a metal ore.
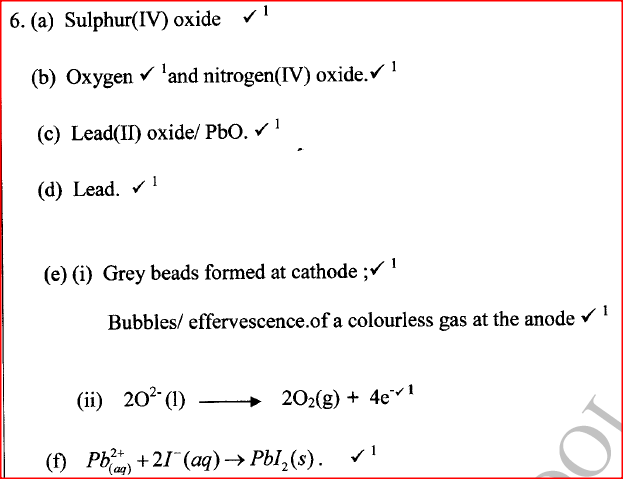
(i) An ore of a metal was roasted in a stream of oxygen. A gas with a pungent smell was formed which turned acidified potassium dichromate(VI) green. (ii) The residue left after roasting was dissolved in hot dilute nitric(V) acid. Crystals were obtained from the solution. (iii) Some crystals were dried and heated. A brown acidic gas and a colourless gas were evolved and a yellow solid remained. (iv) The solid was yellow when cold. (v) The yellow solid was heated with powered charcoal. Shiny beads were formed. Name the: (a) gas formed when the ore was roasted in air. (b) gases evolved when crystals in step (iii) were heated. (c) yellow solid formed in step (iii). (d) shiny beads in step (iv). (e) The yellow solid from procedure (iii) was separated, dried, melted and the melt electrolysed using graphite electrodes. I. Describe the observations made at each electrode. II. Write the equation for the reaction that took place at the anode. (f) Some crystals formed in step (ii) were dissolved in water, and a portion of it reacted with potassium iodide solution. A yellow precipitate was formed. Write an ionic equation for this reaction. (g) To another portion of the solution from (f), sodium hydroxide solution was added drop by drop until there was no further change. Describe the observation made. (h) To a further portion of the solution from (f), a piece of zinc foil was added. I. Name the type of reaction taking place. II. Write an ionic equation for the above reaction.
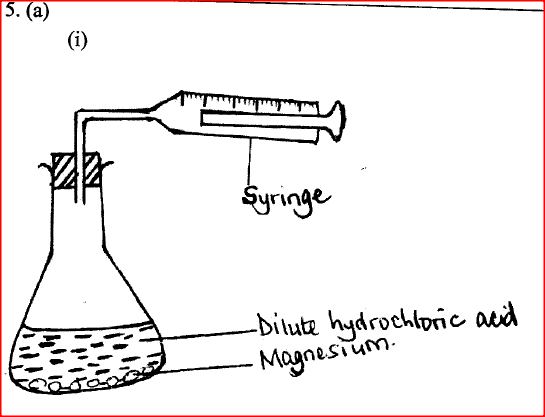
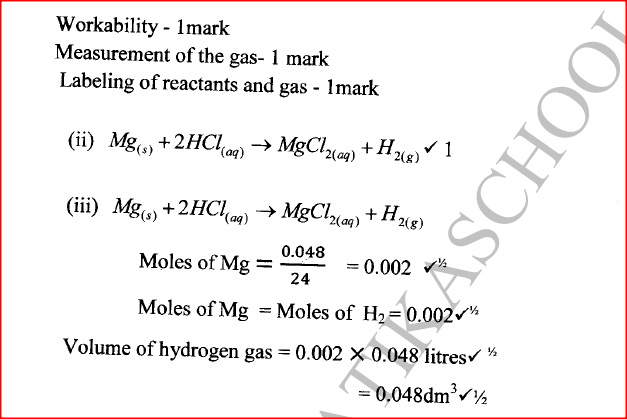
(a) When 0.048 g of magnesium was reacted with excess dilute hydrochloric acid at room temperature and pressure, 50 cm3 of hydrogen gas was collected. (Mg = 24.0; Molar gas volume = 24.0 dm3)
(i) Draw a diagram of the apparatus used to carry out the experiment described above.
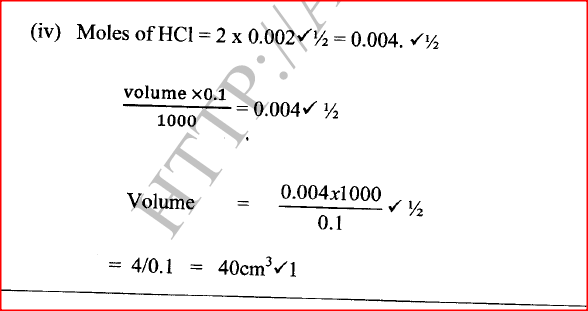
(ii) Write the equation for the reaction. (iii) Calculate the volume of hydrogen gas produced. (iv) Calculate the volume of 0.1 M hydrochloric acid required to react with 0.048 g of magnesium.
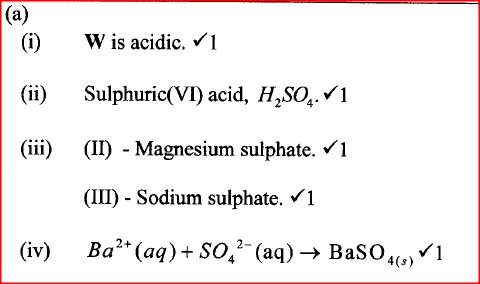
W is a colourless aqueous solution with the following properties:
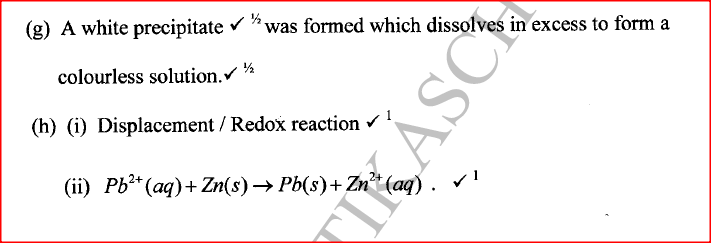
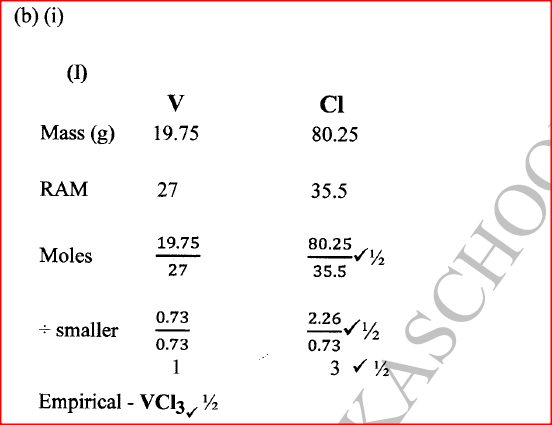
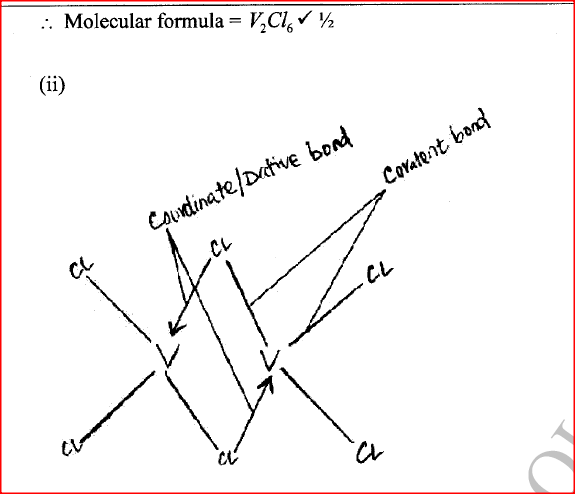
(ii) Give the identity of W. (iii) Name the colourless solution formed in (II) and (III). (iv) Write an ionic equation for the reaction indicated in (V). (b) Element V conducts electricity and melts at 933K. When chlorine gas is passed over heated V, it forms a vapour that solidifies on cooling. The solid chloride dissolves in water to form an acidic solution. The chloride vapour has a relative molecular mass of 267 and contains 19.75% of V. At a higher temperature, it dissociates to a compound of relative molecular mass 133.5. When aqueous sodium hydroxide is added to the aqueous solution of the chloride, a white precipitate is formed which dissolves in excess alkali. (V = 27.0 ; CI = 35.5) (i) Determine the:
(iii) Write an equation for the reaction that form a white precipitate with sodium hydroxide.
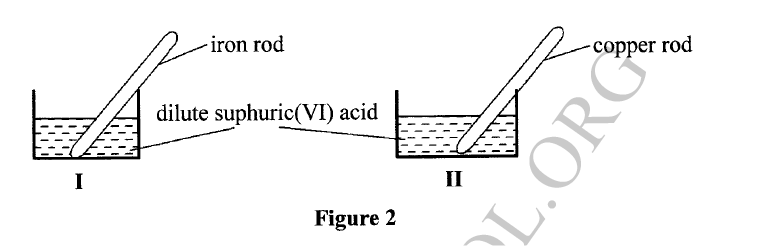
(a) A student used Figure 2 to investigate the action of dilute sulphuric(VI) acid on some metals. Beaker I and II contained equal volumes of dilute sulphuric(VI) acid. To beaker I, a clean iron rod was dipped and to beaker II, a clean copper rod was dipped.
(i) Why was it necessary to clean the metal rods?
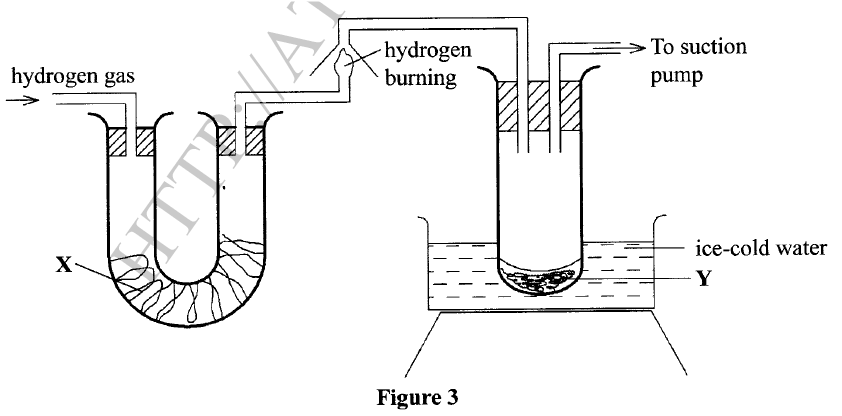
(ii) Describe the observations made in each beaker. Beaker I: Beaker II: (iii) Explain the observations in (a) (ii). (b) Figure 3 shows the apparatus used to burn hydrogen in air. Use it to answer the questions that follow.
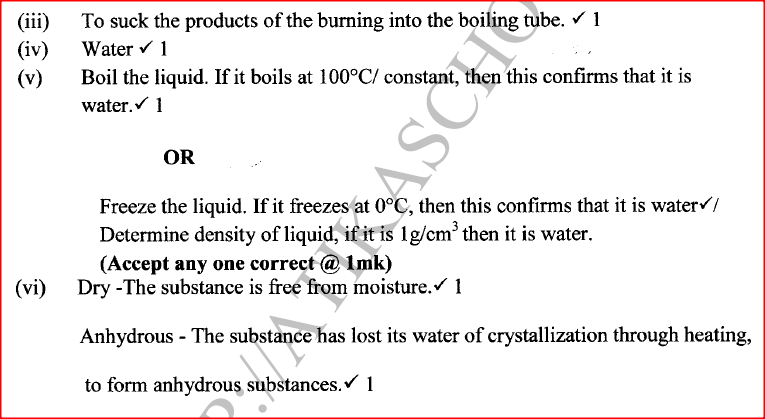
(i)State the role of substance X.
(ii) Give the name of the substance that could be used as X. (iii) State the role of the suction pump. (iv) Name the product Y formed. (v) Give a simple physical test to prove the identity of Y. vi) State the difference between 'dry' and 'anhydrous'.
ANSWERS
(a) (i) To remove oxide layer on the metal.
(ii) Beaker I : . Bubbles of a colourless gas / effervescence ; . Solution turns green; . the size of iron rod decreases Beaker II: . The solution remained colourless. . No bubbles/effervescence
OR
Iron is more reactive than hydrogen hence it reacts with sulphuric(VI) acid to produce hydrogen gas and iron(III) sulphate which is green. Beaker II: Copper is below hydrogen hence no reaction will take place. (b) (i) To dry hydrogen gas. (ii) Calcium oxide /anhydrous calcium chloride /silica gel.
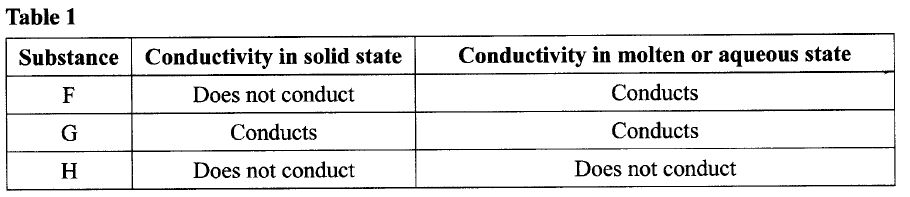
The conductivity of some substances was investigated. The observations made were recorded in
Table 1. Use it to answer the questions that follow.
(a) (i) Identify a substance that is a metal. Give a reason.
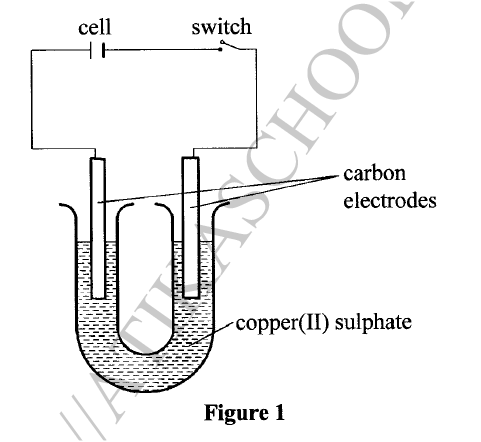
(ii) Substance F does not conduct electricity in solid state but conducts in molten or aqueous state. Explain. (b) Copper(II) sulphate solution was electrolysed using the set up in Figure 1.
(i) State the observations made during electrolysis.
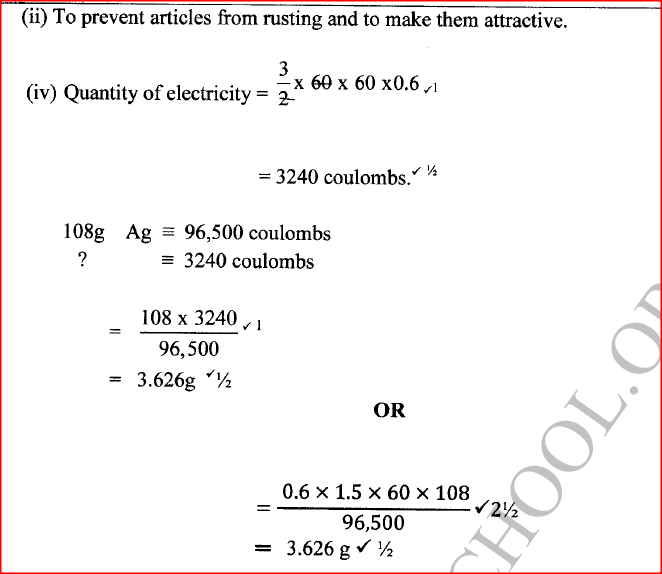
(ii) Write the equation for the reaction that occurs at the anode. (iii) State the expected change in pH of the electrolyte after electrolysis. (c) The experiment was repeated using copper electrodes instead of carbon electrodes. Describe the observations made at each electrode. (d) Electroplating is an important industrial process. What is meant by electroplating. (iii) During electroplating of an iron spoon, a current of 0.6 amperes was passed through aqueous silver nitrate solution for 1 1/2 hours. Calculate the mass of silver that was deposited on the spoon. (Ag = 108.0 ; I F = 96,500 C /mol)
ANSWERS
(a)(i) G — Contains delocalized electrons present in solid and molten state.
(ii) In solid state, the ions are rigidly held in position and cannot move, hence will not conduct. In molten/aqueous state, the ions are mobile and will be able to conduct electric current. (b)(i)The blue electrolyte fades and finally changes from blue to colourless, Effervescence / bubbles of a colourless gas. A brown deposit forms on the cathode.
(c) With copper electrodes:
Anode will go into solution as copper ions hence it decreases in mass/size. Brown deposit forms at the cathode hence the cathode increases in mass. (d) (i) This is the coating of an article / object with another metal by electrolytic method /electrolysis.
(a) Name the homologous series represented by each of the following general formulae.
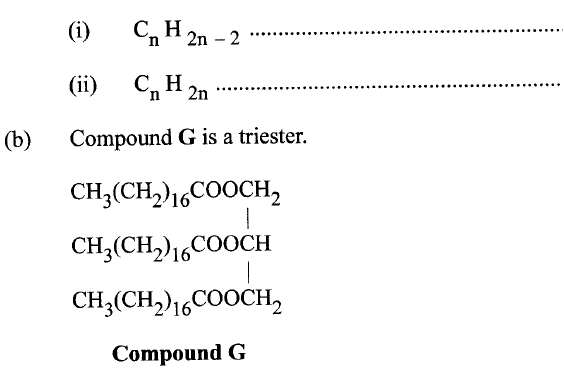
(i) Give the physical state of compound G at room temperature.
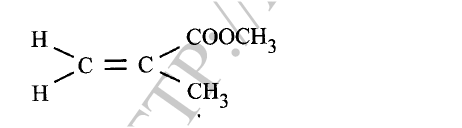
(ii) G is completely hydrolysed by heating with aqueous sodium hydroxide. I Give the structural formula of the alcohol formed. II Write a formula for the sodium salt formed. III State the use of the sodium salt. (c) Ethyne is the first member of the alkyne family. (i) Name two reagents that can be used in the laboratory to prepare the gas. (ii) Write an equation for the reaction. (d) Perspex is an addition synthetic polymer formed from the monomer,
(i) What is meant by addition polymerisation?
(ii) Draw three repeat units of perspex. Give one use of perspex (iv) State two environmental hazards associated with synthetic polymers.
ANSWERS
When an aqueous solution of compound X was mixed with a few drops of bromine water, the colour of the mixture remained yellow.
When another portion of solution X was reacted with acidified potassium dichromate(VI), the colour of the mixture changed from orange to green. (a) What conclusion can be made from the use of: (i) bromine water? (ii) acidified potassium dichromate(VI)? (b) Solution X was reacted with a piece of a metal and a colourless gas was produced. Describe a simple experiment to identify the gas.
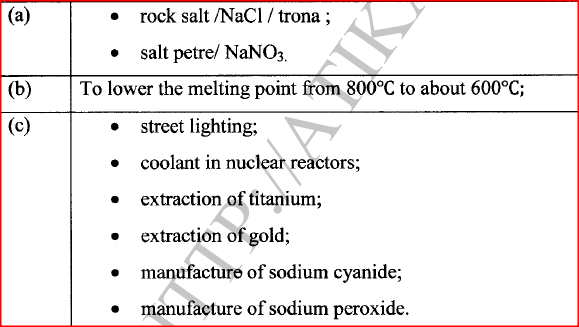
(a) Name two ores in which sodium occurs.
(b) During extraction of sodium using the down's process, calcium chloride is added to the ore. Give a reason for the addition of calcium chloride. (c) State two uses of sodium.
(a) What is meant by the term bleaching?
(b) Write the formula of the bleaching nent formed when chlorine gas reacts with aqueous sodium hydroxide. (c) State the role of chlorine in water treatment.
In terms of structure and bonding, explain why graphite is used as a lubricant in machines.
ANSWERS
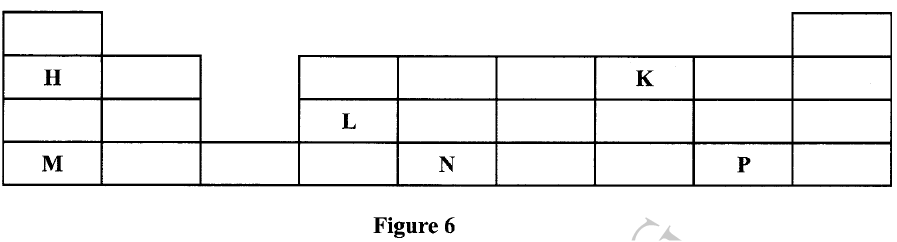
Figure 6 shows part of the periodic table. The letters are not the actual symbols of the elements. Stud it and answer the questions that follow.
(a) Write an equation for the reaction between M and K.
(b) Select the element which can form an ion with a charge of +3. (c) An element J has atomic number 15. Indicate with a tick (✓), on the part of the periodic table the position of J.
Explain how a student can establish whether a liquid sample extracted from a plant is pure.
ANSWERS
(a) What is an inert electrode?
(b) State the products formed when brine is electrolysed using inert electrodes. Anode: Cathode:
ANSWERS
(a)Inert electrode is one which does not participate in the reaction / does not affect the products of electrolysis / does not react;
(b)Anode - chlorine; Cathode - Hydrogen;
The atomic numbers of some elements P, Q, R and S are 6, 8, 12 and 17 respectively.
(a) Draw the dot (•) and cross (X) diagrams for the compounds formed when: (i) R and Q react (ii) P and S react. (b) Explain why the melting point of the compound formed by P and S is lower than that formed by R and Q.
Study the flow chart in Figure 5 and answer the questions that follow.
(a) Identify substances K and L. K:
(b) Name one reagent that can be used to carry out process J.
The following procedure was used to investigate the temperature changes that occur when sodium hydroxide solution is added to dilute hydrochloric acid.
(i) Place the acid in a glass beaker and record its temperature. (ii) Add a known volume of sodium hydroxide solution. (iii) Stir the mixture and record the highest temperature reached. (iv) Repeat steps (ii) and (iii) with different volumes of sodium hydroxide solution. (a) State two factors that must be kept constant in this experiment (b) Explain how the use of a polystyrene cup will affect the results.
ANSWERS
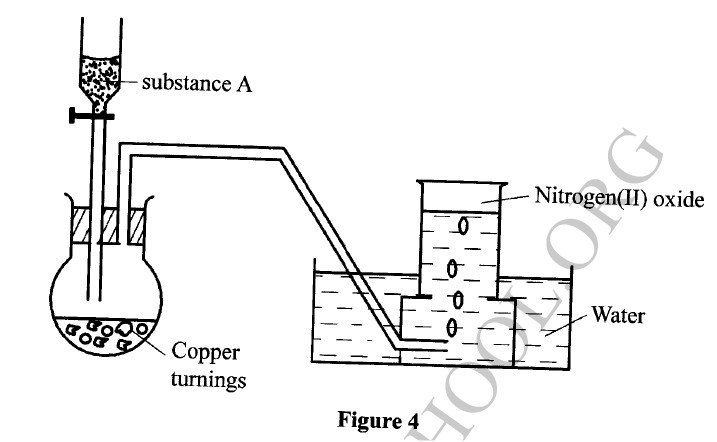
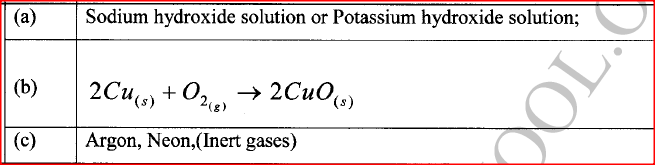
The set-up in Figure 4 can be used to prepare nitrogen (II) oxide. Use it to answer the questions that follow.
(a) Name substance A
(b) When the gas jar containing nitrogen (II) oxide is exposed to air, a brown color is observed. Explain. (c) Write an equation for the reaction which occurred in the flask.
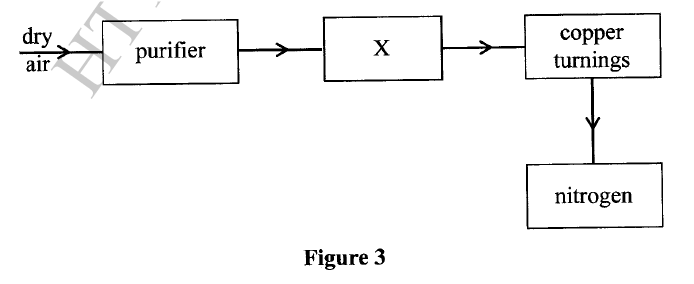
The flow chart in Figure 3 shows the process of obtaining a sample of nitrogen gas. Study it and answer the questions that follow.
(a) Identify X
(b) Write an equation for the reaction with heated copper turnings. (c) Name an impurity in the sample of nitrogen gas.
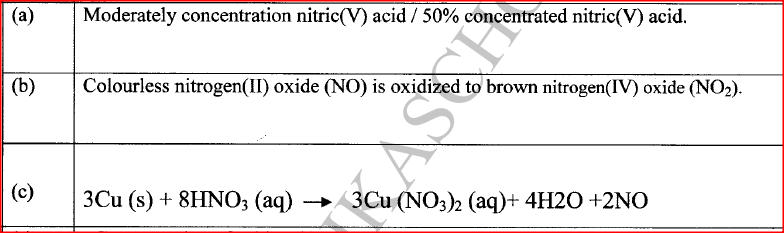
In an experiment, concentrated nitric(V) acid was reacted with iron(II) sulphate. State and explain the observations made.
ANSWERS
The mixture changed from green to yellow / formation of a brown gas;
Iron(II) ions is oxidized by nitric(V) acid to Iron(III) ions / nitric(V) acid is reduced to nitrogen(1I) oxide which is oxidized by oxygen to nitrogen(IV ) oxide.
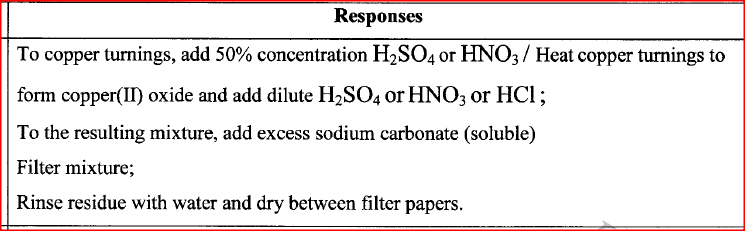
Starting with copper, describe how a pure sample of copper(II) carbonate can be prepared.
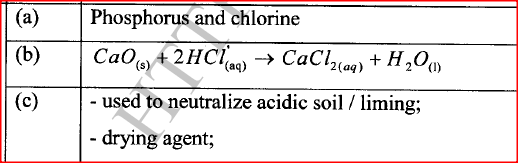
Using the elements chlorine, calcium and phosphorus:
(a) Select elements that will form an oxide whose aqueous solution has a pH less than 7. (b) Write an equation for the reaction between calcium oxide and dilute hydrochloric acid. (c) Give one use of calcium oxide.
Explain the observation made when chlorine gas is passed through a solution of potassium iodide.
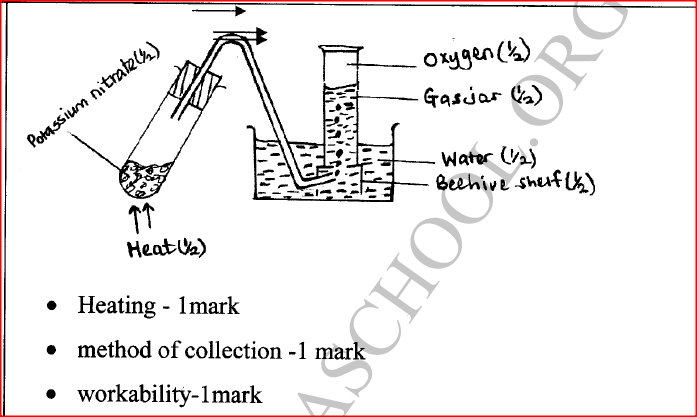
Potassium nitrate liberates oxygen gas when heated. Draw a diagram of a set-up that shows heating of potassium nitrate and collection of oxygen gas.
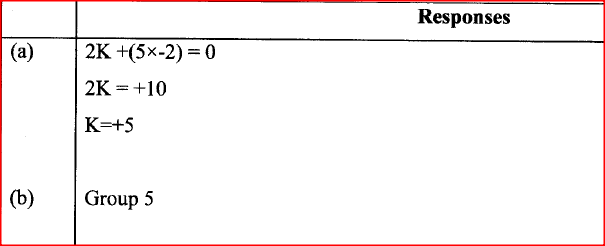
An oxide of element K has the formula K2O5.
(a) Determine the oxidation number of K. (b) To which group of the periodic table does K belong? |
Chemistry Topics
All
Archives
December 2024
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
|
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |
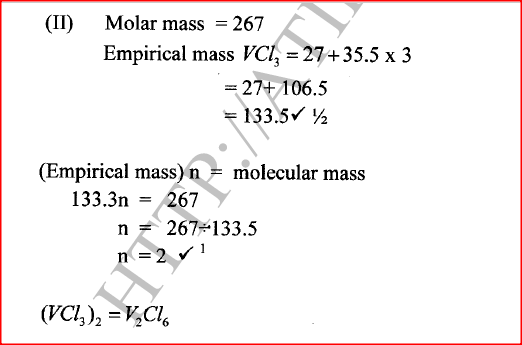

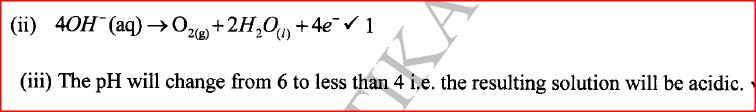


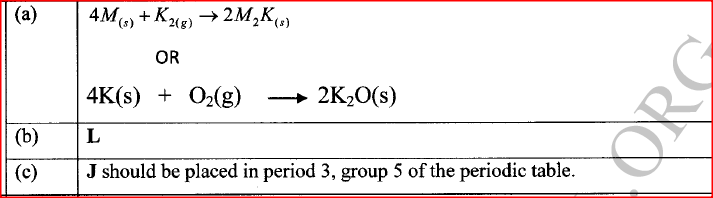






 RSS Feed
RSS Feed

