|
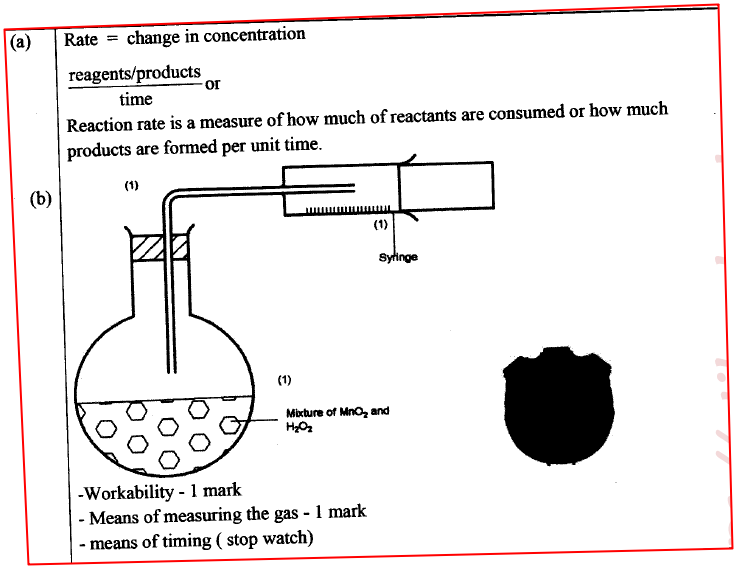
(a) What is meant by rate of reaction. (1 mark)
(b) In the space provided, sketch the diagram of a set-up that can be used to determine the rate of reaction between manganese(IV) oxide and hydrogen peroxide. (3 marks)
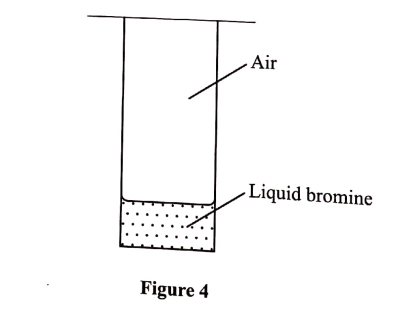
(e) A student placed a small amount of liquid bromine at the bottom of a sealed gas jar of air as shown in Figure 4.
(i) Describe what will be observed: (1 mark)
I. after two minutes . II. after 30 minutes
(ii) Use the Kinetic theory to explain the observations: (2 marks)
I. after 2 minutes . II. after 30 minutes
(d) Some plants have seeds that contain vegetable oil.
0 Comments
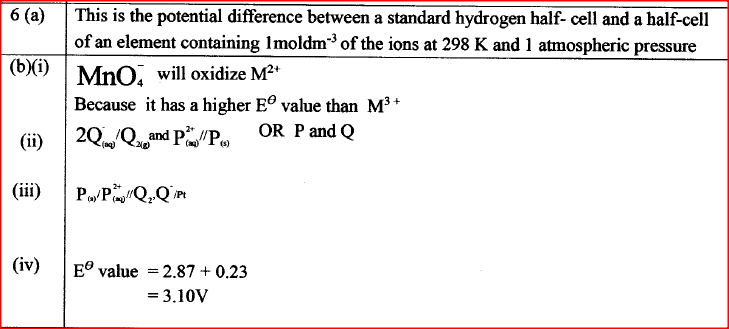
(a) What is meant by standard electrode potential of a an element? (1 mark)
(b) use the standard electrode potentials given below to answer the questions that follow.
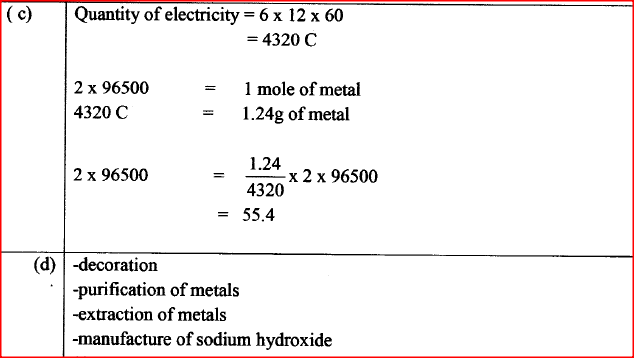
(i) State whether acidified MnO4- can oxidise M2+. Give a reason. (2 marks) (ii) Select two half-cells which when combined will give the highest e.m.f. (1 mark) (iii) Write the cell representation for the cell formed in b (ii). (I mark) (iv) Calculate the E0 value for the cell formed in b (iii). (2 marks) (c) A mass of 1.24g of a divalent metal was deposited when a current of 6A was passed through a solution of the metal sulphate for 12 minutes. Determine the relative atomic mass of the metal.
(1 Faraday = 96,500 C mol-1 (3 marks)
(d) State two applications of electrolysis. (I mark)
(a) What ¡s meant by molar heat of neutralisation? (1 mark)
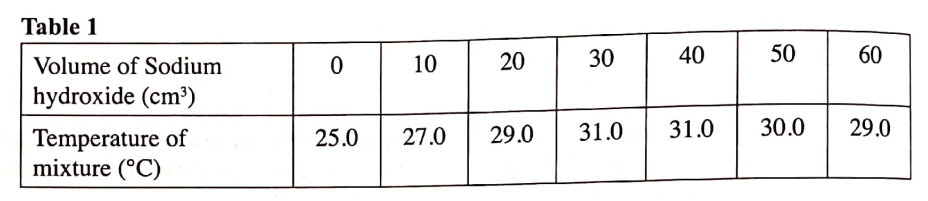
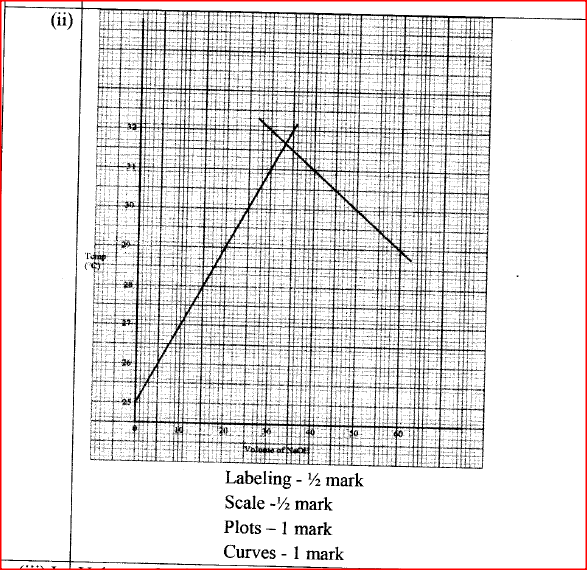
(b) In an experiment to determine the molar heat of neutralisation, 50 cm3 of 1M hydrochloric acid was neutralised by adding 10 cm3 portions of dilute sodium hydroxide. During the experiment, the data in Table 1 was obtained.
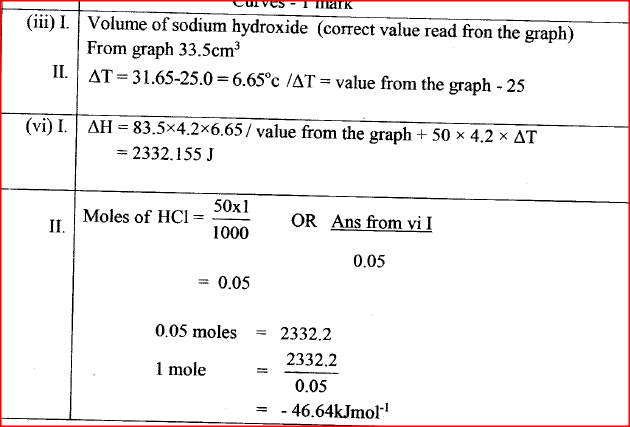
(iii) Determine from the graph the: I. volume of sodium hydroxide which completely neutralises 50 cm3 of 1M hydrochloric acid. (1 mark) II. change in temperature,△T, when complete neutralisation occurred. (1 mark) (iv) Calculate: I. the heat change, △H when complete neutralisation occurred. (Specific heat capacity = 4.2 Jg-1K-1, density of solution 1.0 gcm-3) (2 marks) II. molar heat of neutralisation of hydrochloric acid with sodium hydroxide. (1 mark) (v) How would the value of molar heat differ if 50 cm3 of 1M ethanoic acid was used instead of 1M hydrochloric acid? Give a reason. (2 marks)
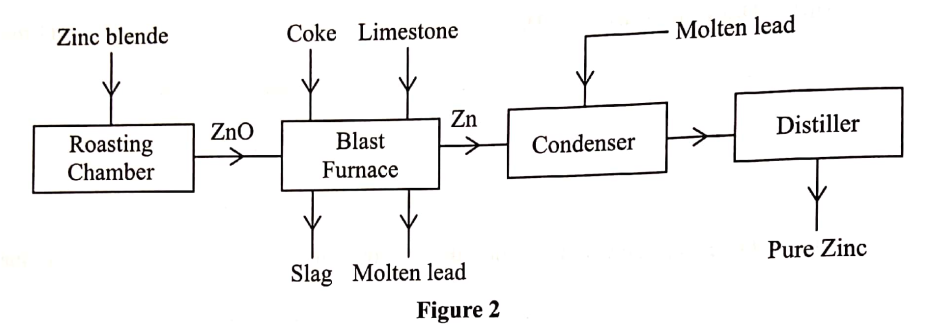
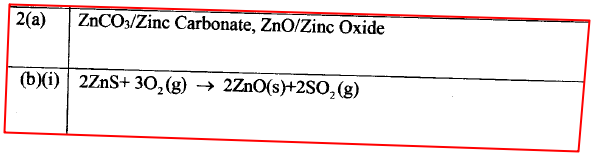
(a) Zinc occurs mainly as zinc blende. Name one other ore from which Zinc can be extracted. (1 mark)
(b) The flow chart in Figure 2 shows the various stages in the extraction of zinc metal. Study it and answer the questions that follow.
(c) Explain the observations made when zinc metal is added to hot sodium hydroxide. (2 marks)
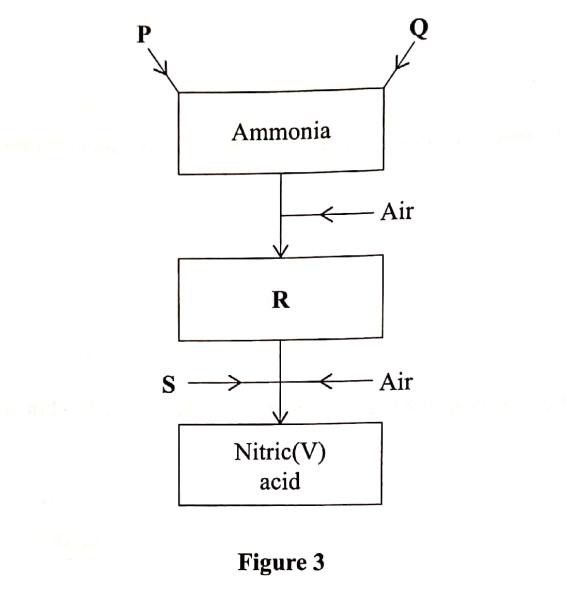
Figure 3 is a flow chart that shows the process that occurs in the manufacture of nitric (V) acid.
(a) Name substance P, Q, R and S.
To obtain substance R, ammonia is heated at 9000C in the presence of air and a catalyst.
The product is then cooled in air.
(e) When ammonia ¡s reacted with nitric(V) acid, it produces a nitrogenous fertiliser.
(a) Explain the following observations:
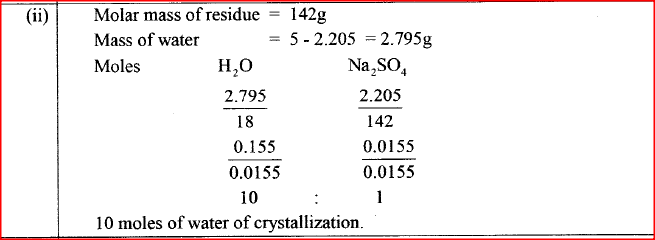
(b) A sample of water is suspected to contain aluminium ions (AI3+) . Describe a laboratory experiment that can be carried out to show that AI3+ ions are present in the water sample. (3 marks) (c) In an experiment to determine the number of moles of water of crystallisation of a hydrated compound, Na2S04. X H2O, 5g of the compound were heated strongly to a constant mass.
1.
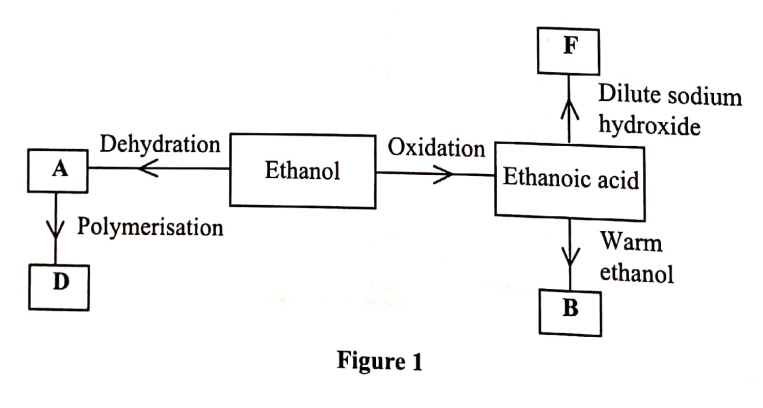
(a) Alkanes are said to be saturated hydrocarbons.
(b) When the alkane, hexane, is heated to high temperature, one of the products is ethene.
Related Chemistry Questions and Answers on Organic Chemistry II Form 3 Level
Name an appropriate apparatus that is used to prepare standard solutions in the laboratory. (1 mark)
Expected Response
volumetric flask
Related Chemistry Questions and Answers on Simple Classification of Substances Form 1 Level
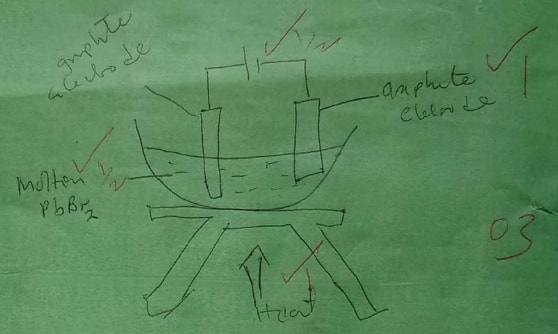
Draw in the space provided a labelled diagram of the set-up of the apparatus that can be used to electrolyse molten Lead (II) bromide. (3 marks)
Related Chemistry Questions and Answers on Effects of Electric Current on Substances Form 2 Level
When burning magnesium ribbon is introduced into a gas jar full of nitrogen, it continues to burn producing a greenish yellow powder.
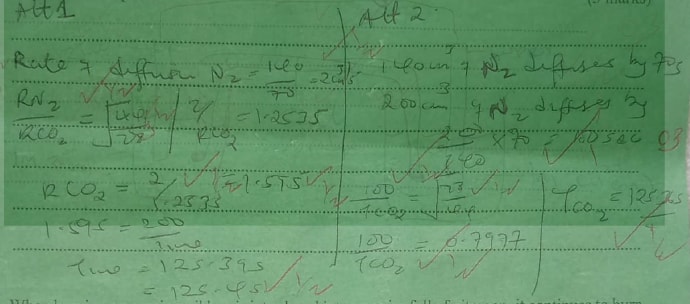
140cm3 of nitrogen gas diffuses through a membrane in 70 seconds. Flow long will it take 200cm3 of carbon(IV) oxide gas to diffuse through the same membrane under the same conditions of temperature and pressure. (3 marks)
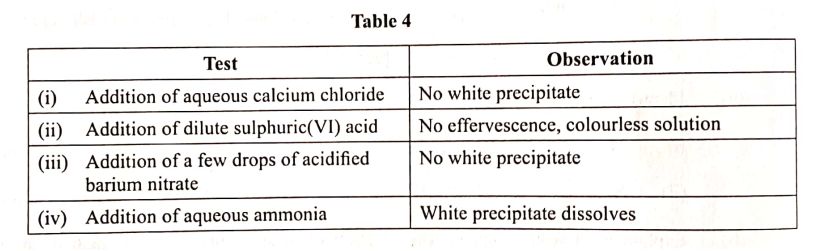
Chemical tests were carried out on separate samples of water drawn from the same source. The observations made were recorded as shown in Table 4.
State the inferences made in reactions:
Starting with copper turnings. describe how a sample of copper(II) sulphate crystals can be prepared in the laboratory. (3 marks)
are isotopes of element X. They occur naturally in the ratio of 9:1 respectively.
Calculate the relative atomic mass of element X. (2 marks)
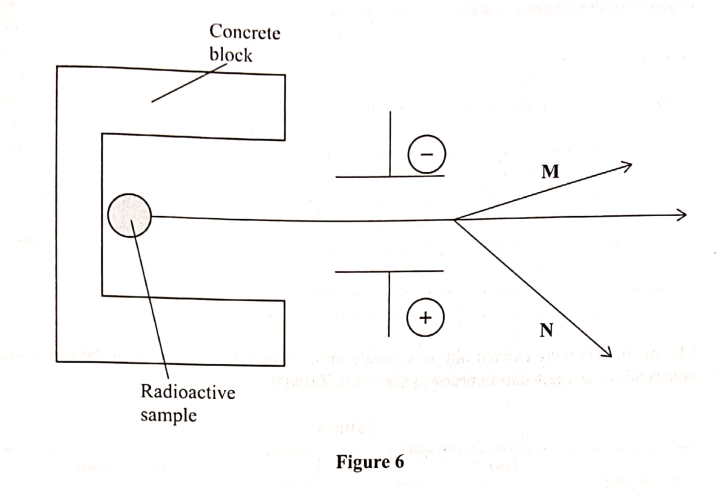
The diagram in Figure 6 Shows radiations emitted by a radioactive sample.
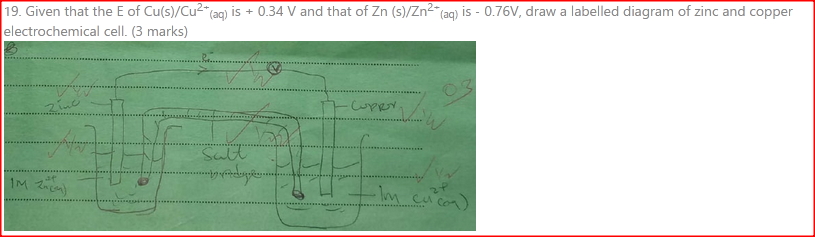
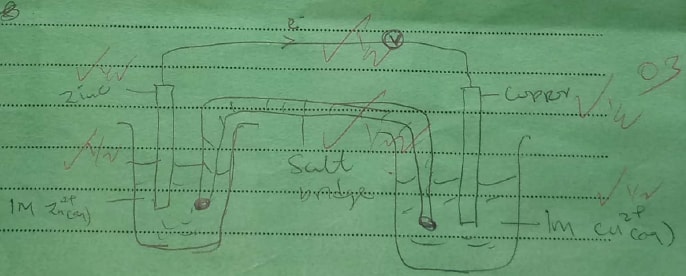
Given that the E° of Cu(s) /Cu2+(aq) is +0.34 V and that of Zn (s)/Zn2+(aq) is —0.76 V, draw a labelled diagram of zinc and copper electrochemical cell. (3 marks)
During laboratory preparation of carbon(IV) oxide gas, dilute hydrochloric acid was added to substance L in a conical flask.
Study the information in Table 3 and use it to answer the questions that follow.
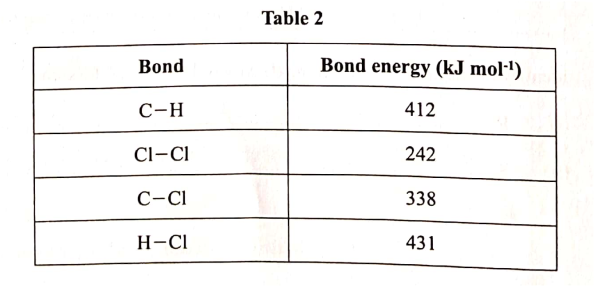
Use the information in Table 2 to answer the questions that follow.
(a) State what is meant by heat of reaction. (1 mark)
(b) Calculate the heat change when one mole of methane reacts completely with excess chlorine in the presence of UV light. (2 marks)
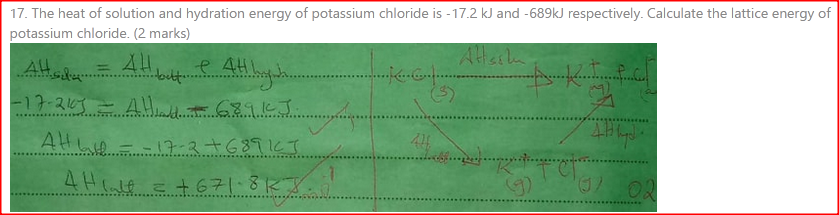
The heat of solution and hydration energy of potassium chloride is —17.2kJ and —689kJ respectively. Calculate the lattice energy of potassium chloride. (2 marks)
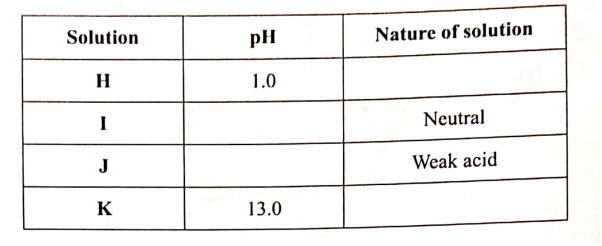
(a)Complete the following table. (2 marks)
(b) Explain why a solution of ammonia in methylbenzene has no effects on red litmus paper while in aqueous ammonia red litmus paper turns blue. (1 mark)
5g of calcium carbonate was strongly heated to a constant mass. Calculate the mass of the solid residue formed (Ca=40 0; C= 12.0; 0= 16.0). (2 marks)
During laboratory preparation of oxygen, manganese(IV) oxide is added to reagent H.
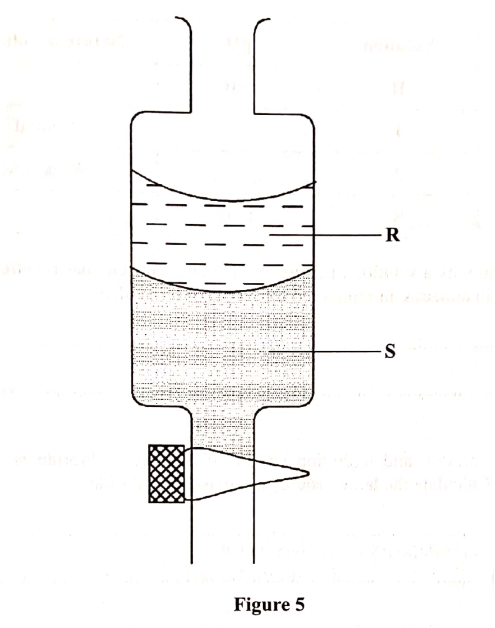
Figure 5 shows an apparatus used to separate a mixture of water and hexene.
A boiling tube filled with chlorine water was inverted in a trough containing the same solution and the set-up left in sunlight for about 2 hours.
|
Chemistry Topics
All
Archives
December 2024
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
|
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |

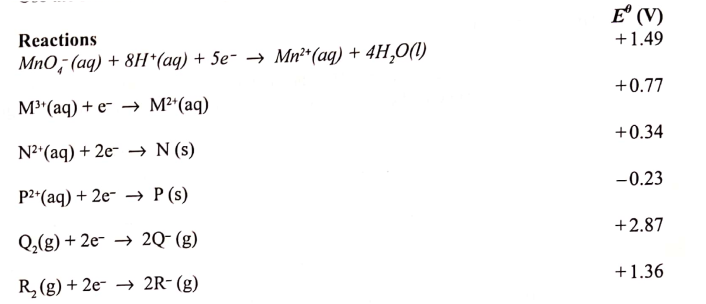
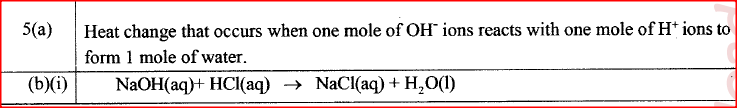







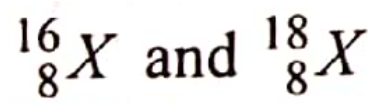






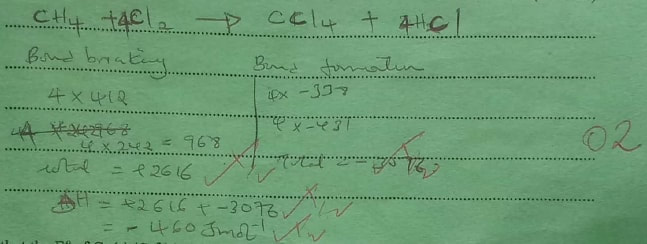








 RSS Feed
RSS Feed

