|
These are chemistry questions and answers categorized according to topics, papers i.e. Paper 1 and 2, Levels i.e. form 1 to form 4, kcse year the examination was done and section A or B
Select topic/category to open topical questions from that particular option provided. Chemistry Topics
0 Comments
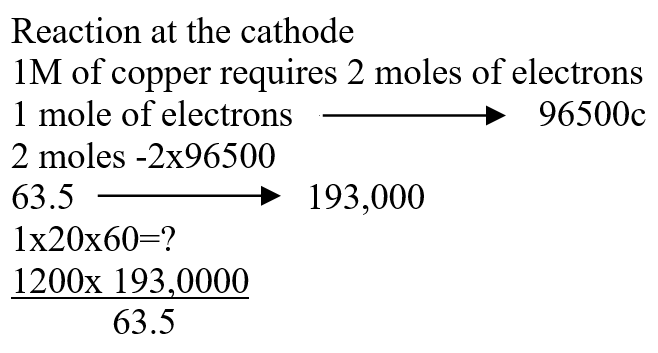
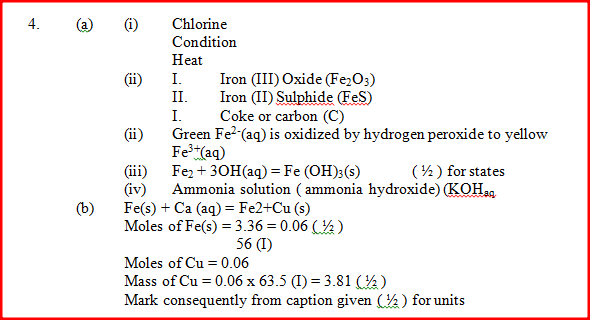
a) Name one chief ore of copper and give its formula (2mks)Copper pyrites -CuFeS2 (any one 1mk) Cuprite – Cu2O2 Calculate the mass of copper that would be deposited on the cathode when a steady current of one ampere flows for 20 minutes through copper (II) sulphate solution (Cu = 63.5; Faraday Constance = 96500Cmol-1) (3mks)
(a) Name two ores of iron.
(b) Describe how the amount of iron in a sample of iron(III) oxide can be determined.
ANSWERS
(a) Haematite;
Magnetite; Siderite. (b) Weigh the iron (III) oxide together with a crucible; Heat the Iron(III) oxide and coke to a constant mass; Cool and re-weigh residue and crucible The difference in mass is weight of the iron.
The following steps were used to analyse a metal ore.
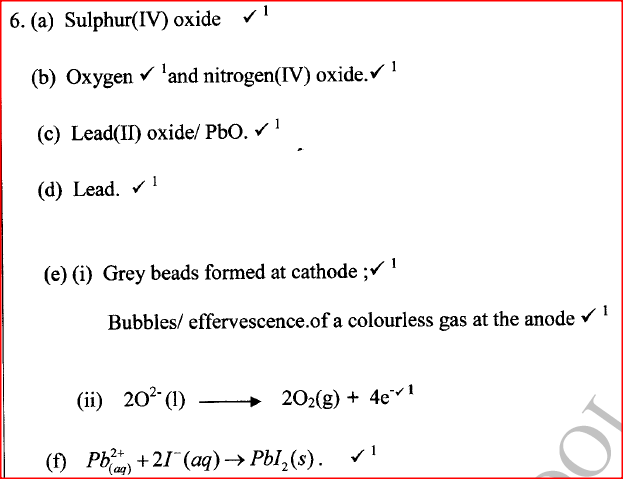
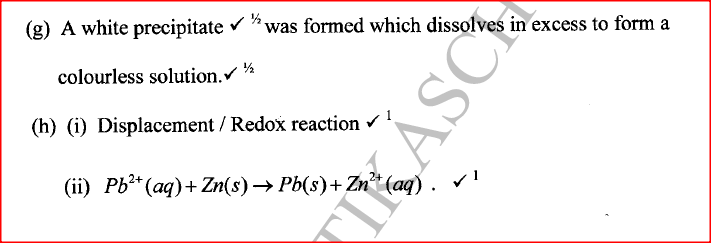
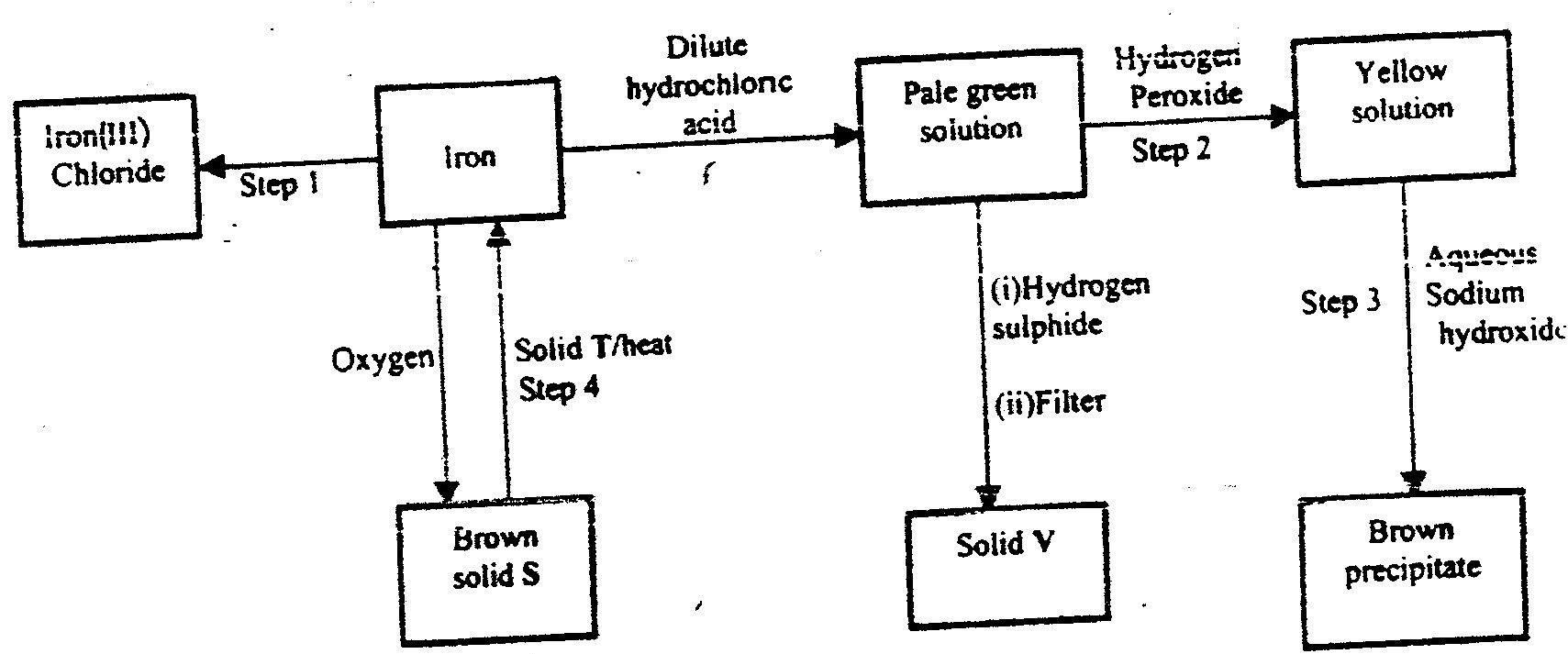
(i) An ore of a metal was roasted in a stream of oxygen. A gas with a pungent smell was formed which turned acidified potassium dichromate(VI) green. (ii) The residue left after roasting was dissolved in hot dilute nitric(V) acid. Crystals were obtained from the solution. (iii) Some crystals were dried and heated. A brown acidic gas and a colourless gas were evolved and a yellow solid remained. (iv) The solid was yellow when cold. (v) The yellow solid was heated with powered charcoal. Shiny beads were formed. Name the: (a) gas formed when the ore was roasted in air. (b) gases evolved when crystals in step (iii) were heated. (c) yellow solid formed in step (iii). (d) shiny beads in step (iv). (e) The yellow solid from procedure (iii) was separated, dried, melted and the melt electrolysed using graphite electrodes. I. Describe the observations made at each electrode. II. Write the equation for the reaction that took place at the anode. (f) Some crystals formed in step (ii) were dissolved in water, and a portion of it reacted with potassium iodide solution. A yellow precipitate was formed. Write an ionic equation for this reaction. (g) To another portion of the solution from (f), sodium hydroxide solution was added drop by drop until there was no further change. Describe the observation made. (h) To a further portion of the solution from (f), a piece of zinc foil was added. I. Name the type of reaction taking place. II. Write an ionic equation for the above reaction.
(a) Name two ores in which sodium occurs.
(b) During extraction of sodium using the down's process, calcium chloride is added to the ore. Give a reason for the addition of calcium chloride. (c) State two uses of sodium.
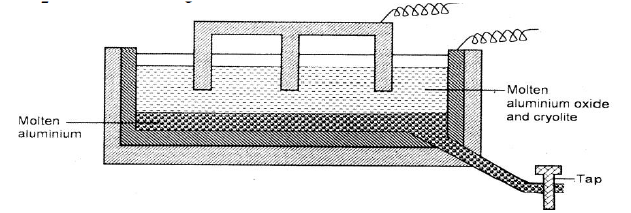
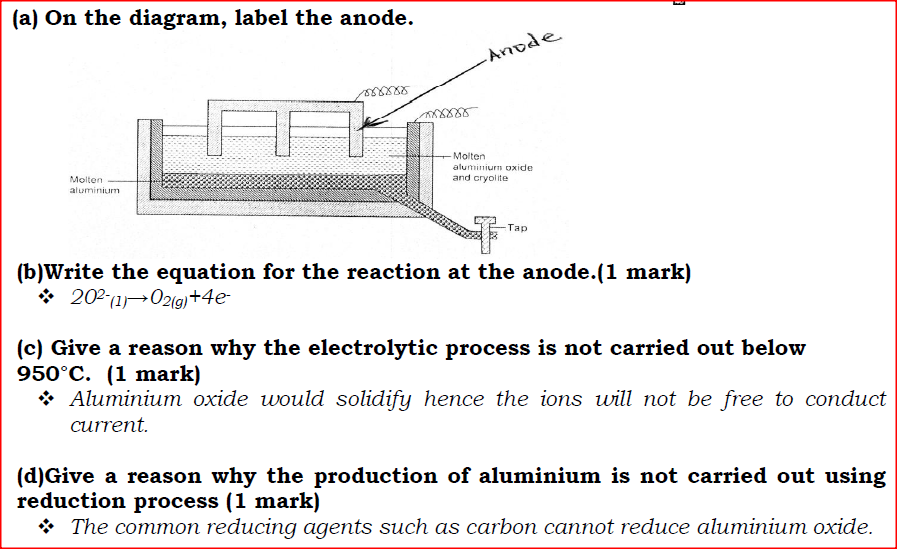
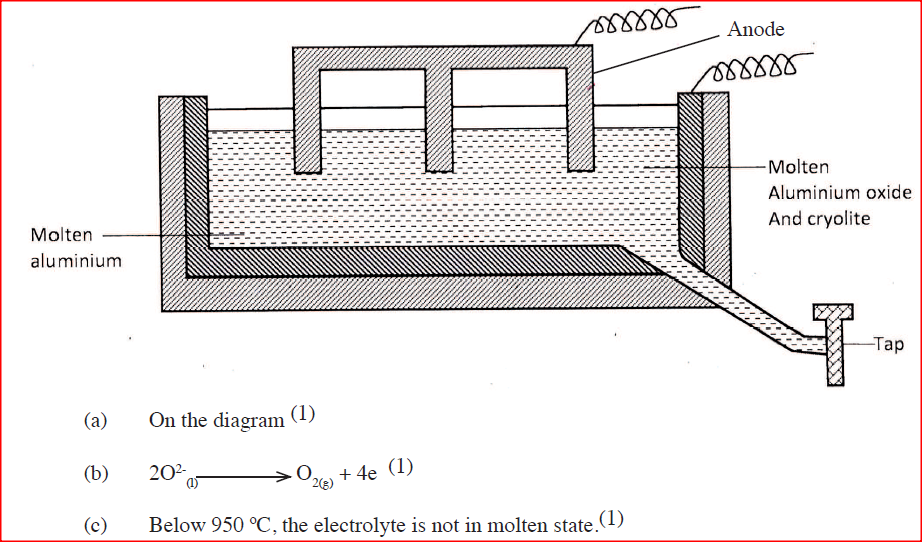
The diagram below represents a set up of an electrolytic cell that can be used in the production of aluminium.
(a) On the diagram, label the anode.
(b)Write the equation for the reaction at the anode. (c) Give a reason why the electrolytic process is not carried out below 950°C. (d)Give a reason why the production of aluminium is not carried out using reduction process (e)Give two reasons why only the aluminium ions are discharged (f)State two properties of duralumin that makes it suitable for use in aircraft industry. (g)Name two environmental effects caused by extraction of aluminium.
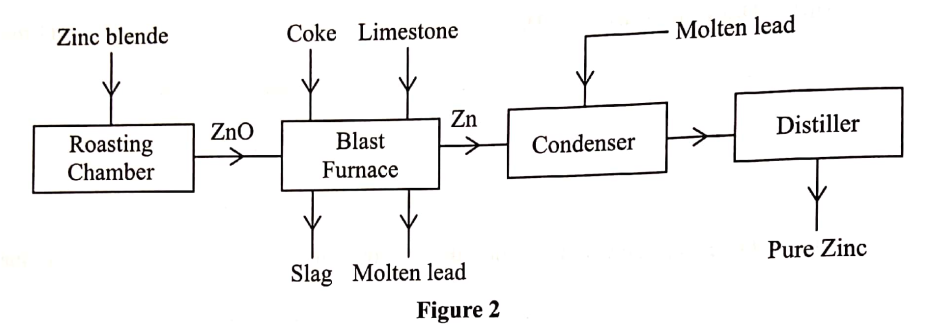
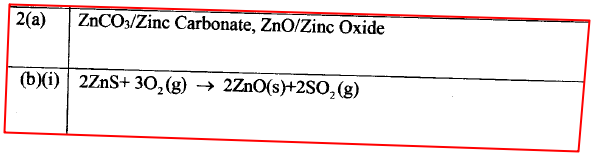
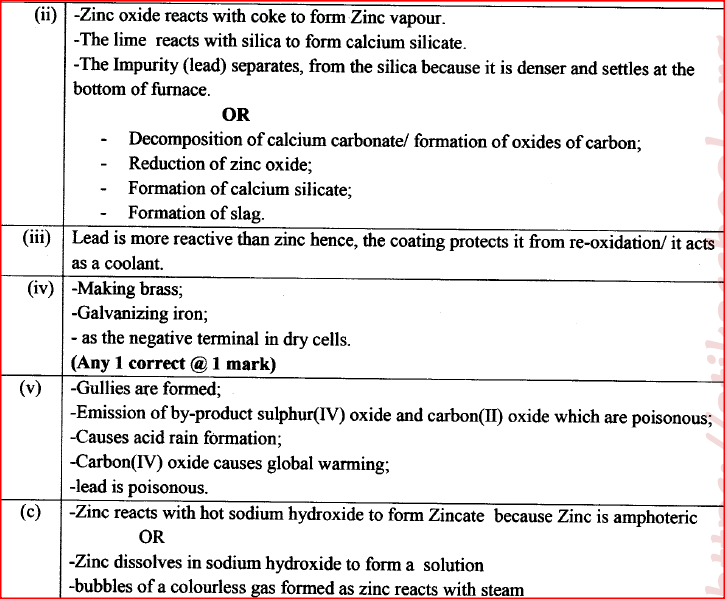
(a) Zinc occurs mainly as zinc blende. Name one other ore from which Zinc can be extracted. (1 mark)
(b) The flow chart in Figure 2 shows the various stages in the extraction of zinc metal. Study it and answer the questions that follow.
(c) Explain the observations made when zinc metal is added to hot sodium hydroxide. (2 marks)
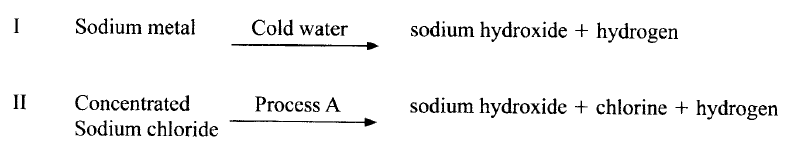
Sodium hydroxide can be prepared by the following methods; I and II
(a) Name one precaution that needs to be taken in method I.
(b) Give the name of process A. (c) Give one use of sodium hydroxide.
ANSWERS
(a) Small piece of sodium metal (pea size) with a lot of water
Perform the experiment wearing goggles. (b) Electrolysis (c) Manufacture of paper (soften), soaps and detergents Fractional distillation of liquid air Extraction of aluminium metal Manufacture of bleaching agents eg NaOCl paper, textiles, oil refinery Making herbicides on weed killers Textile industry to soften
Aluminium is both malleable and ductile.
(a)What is meant by? (i) Malleable: (ii)Ductile (b)State One use of aluminium based on: (i)malleability (ii)ductility
ANSWERS
(a) (i) Can be hammered into sheets.
(ii)Can be drawn into wires. (b)(i) Making of sufurias/ motor vehicle parts/ aeroplane parts,window / door flames, cups, plates, packaging materials, pans, making sheets/ roof. (ii)electricity cables/ wires.
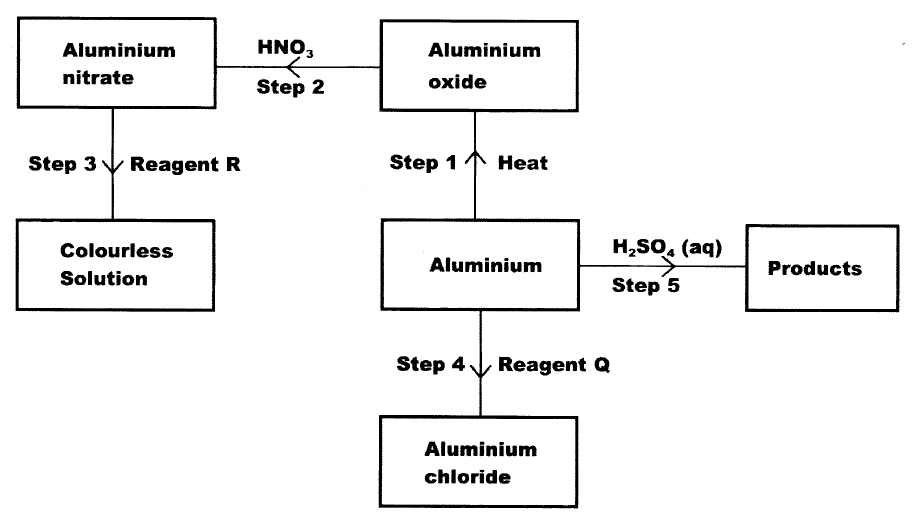
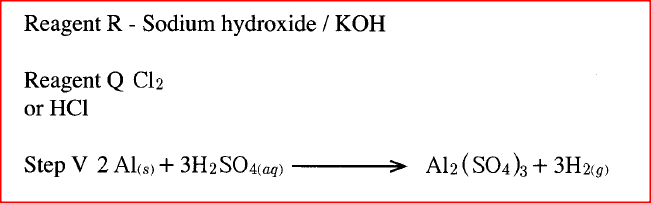
The flow chart below shows various reactions of aluminium metal. Study it and answer the questions that follow
a) i) Other than water, name another reagent that could be R
ii) Write the formula of reagent Q b) Write an equation or the reaction in step 5.
(a) Name the raw material from which sodium is extracted.
(b) Give a reason why sodium is extracted using electrolysis. (c) Give two uses of sodium metal.
ANSWERS
(a)Brine (NaCl)
(b) Sodium is very reactive (use electrolysis) More reactive than carbon. (c) Uses Sodium lamps, coolant in nuclear reactors Sodium cyanide, sodium amalgam Na202 , Extraction of titanium,
The diagram below represents a set up of an electrolytic cell that can be used in the production of aluminium
(a) One the diagram, label the anode
(b) Write the equation for the reaction at the anode (c) Give a reason why the electrolytic process is not carried out below 950°C (d) Give a reason why the production of aluminium is not carried out using reduction process (e) Give two reasons why only the aluminium ions are discharged (f) State two properties of duralumin that makes it suitable for use in aircraft industry (g)Name two environmental effects caused by extraction of aluminium
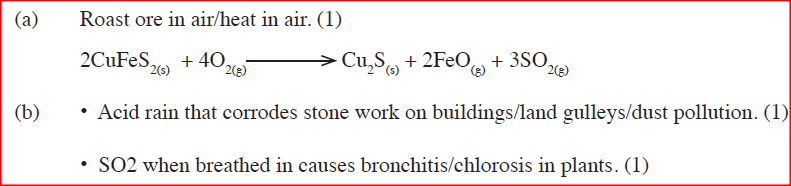
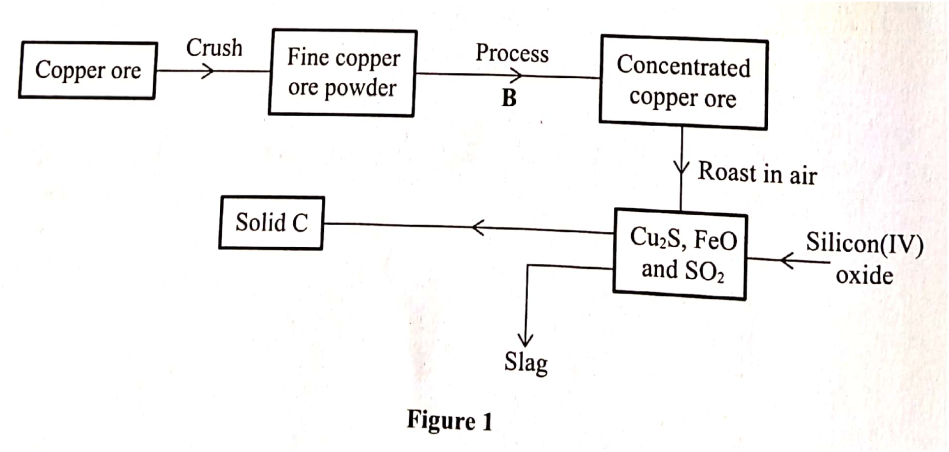
One of the ores of copper has formula, CuFeS2.
(a) Describe how iron in the ore is removed during concentration of copper metal. (b) State two environmental problems associated with extraction of copper metal.
The flow chart in Figure 1 represents some stages in the extraction of copper metal. Study it and answer the questions that follow.
Identify:
a) Name two ores from which copper is extracted
b) During extraction of copper metal, the ore is subjected to froth flotation. Give a reason why this process is necessary. c) Name one alloy of copper and state its use.
ANSWERS
(a) Copper pyrites chalcocite, malachite
(b) To concentrate the ore (c) Brass Batteries
Describe a simple laboratory experiment that can be sued to distinguish between sodium and sulphide and sodium carbonate.
Expected Answer
Add dilute acid (e.g. HCI or H2SO4) to each substances separately. If Na2S, colourless gas, smell of rotten eggs
Aluminium is both malleable and ductile,
(a) What is meant by? (i) malleable; (ii) ductible. (b) State one use of aluminium based on: (i) malleability (ii) ductility
ANSWERS
(a) (i) Malleable - Can be hammed into sheets
(ii) Ductile - Can be drawn into wires (b) (i) Saucepans (ii) Electrical transmission lines
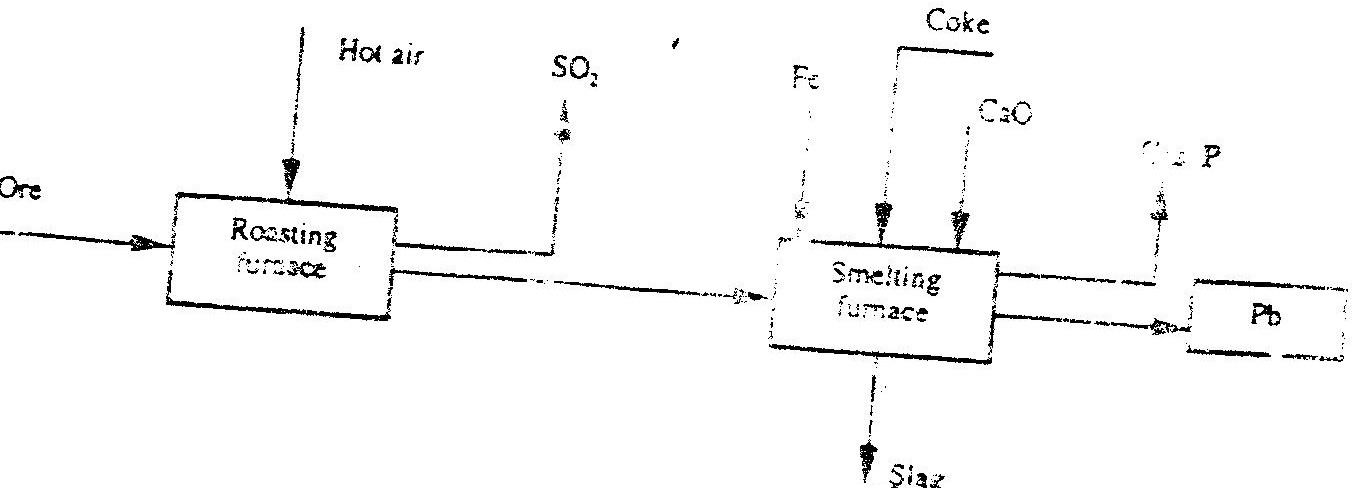
The flow chart below illustrate the industrial extraction of lead metal. Study it and answer the questions that follow
(a)
(c) State one use of lead other than the making of lead pipes Related Chemistry Questions and Answers on Metals Form 4 Level
a) The flow chart below shows a sequence of reactions starting with.
Study and answer the questions that follow.
Cu = 63.5, Fe = 56.0
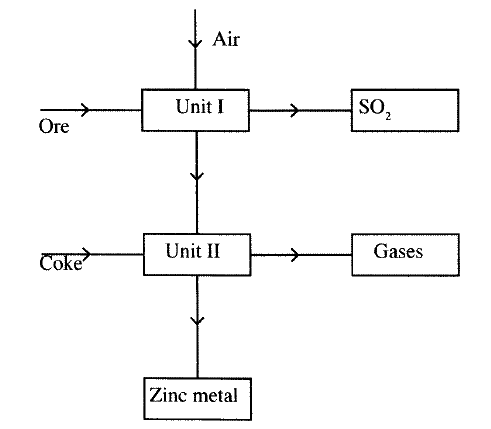
The flow chart below shows some processes involved in the extraction of zinc metal:
a) Name oneore from which zinc is extracted.
b) Write the equation of the reaction taking place in unit II c) Name two uses of zinc metal.
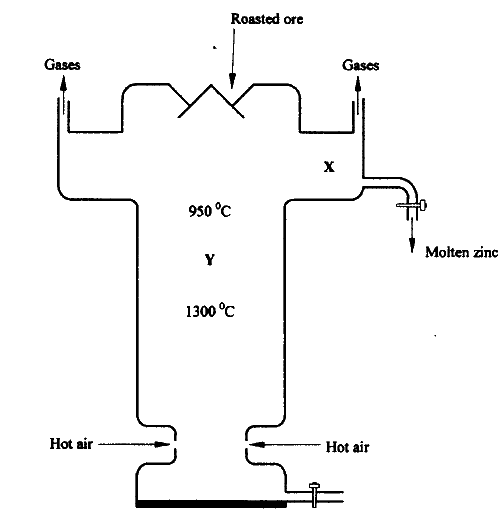
The melting and boiling points of zinc are 419°C and 907°C respectively. One of the ores of zinc blende. To extract zinc, the ore is first roasted in air before feeding it into a furnace.
a. i) Write the formula of the main zinc compound in zinc blende. ii) Explain using an equation why it is necessary to roast the ore in air before introducing it into the furnace b. The diagram below shows a simplified furnace used in the extraction of zinc. Study it and answer the questions that follows:
i) Name two other substance that are also introduced into the furnace together with roasted ore.
ii) The main reducing agent in the furnace is carbon(II) oxide. Write two equations showing how it is formed. iii) In which physical state is zinc at point Y in the furnace? Give a reason iv) Suggest a value for the temperature at point X in the furnace. Give a reason. v) State and explain one environmental effect that may arise from the extraction of zinc from zinc blende vi) Give two industrial uses of zinc.
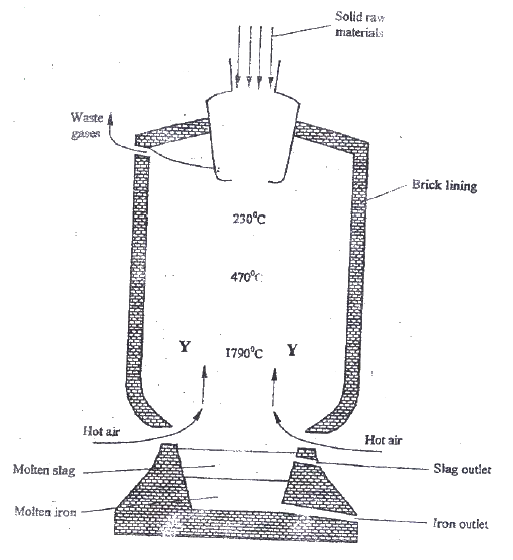
Iron is obtained from haematite using a blast furnace shown if figure 5 below.
a) Four raw materials are required for the production of iron. Three of these are iron oxide, hot air and limestone
Give the name of the fourth raw material b) Write an equation for the reaction in which carbon (IV) oxide is converted into carbon (II) oxide. c) Explain why the temperature in the region marked Y is higher than that of the incoming hot air. d) State one physical property of molten slag other than density that allows it to be separated from molten iron as shown in figure 5. e) One of the components of the waste gases is Nitrogen (IV) oxide describe the adverse effects it has on the environment. f) Iron from the blast furnace contains about 5% carbon i) Describe how the carbon content is reduced. ii) Why is it necessary to reduce the carbon content?
ANSWERS
(a) Coke/ coal/ Charcoal/ Carbon
(b) C(s) + CO2 (g) → 2 CO(g) (c) The reaction between coke/ coal and the hot air is highly exothermic (d) Slog is immiscible with molten iron (e) Nitrogen (iv) oxide gas forms acid rain. Which corrodes metallic materials and destroys vegetation the environment. (f) (i) By passing/ blowing oxygen into molten iron which converts carbon into carbon (iv) Oxide (ii) To increase the tensile strength/ making the iron less brittle/ making it more malleable / making it more ductile.
Aluminium metal is a good conductor and is used for overhead cables. State any other two properties that make aluminium suitable for this use.
ANSWERS
(a)(i)Cryolite
(ii)Electrolysis (b)Good conductor Does not rust Malleable Light High M.P Does not corrode easily
Give one advantage and one disadvantage of using petrol containing tetraethyl lead in motor vehicles.
Expected Response
Advantage
|
Chemistry Topics
All
Archives
December 2024
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
|
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |







 RSS Feed
RSS Feed

