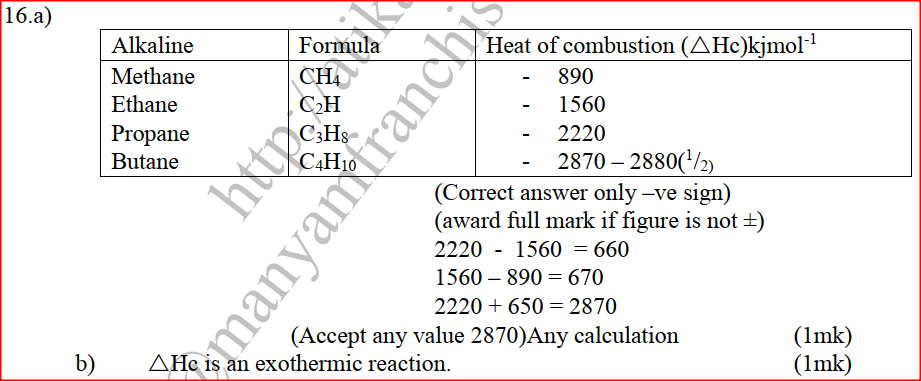
|
A hydrocarbon slowly decolorizes bromine gas in the presence of sunlight but does not decolourise acidified potassium permanganate
Name and draw the structural formula of the fourth member of the series to which the hydrocarbon belongs.
Expected Response
0 Comments
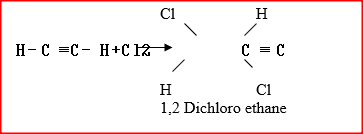
a) Draw and name the structure of the compound formed when one mole of ethyne reacts with one mole of hydrogen bromide.
b) Draw the structures of the alkynes whose molecular formula is C4H6
The formula given below represents a portion of a polymer Give:
Expected Response
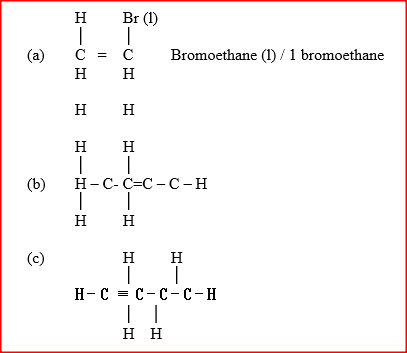
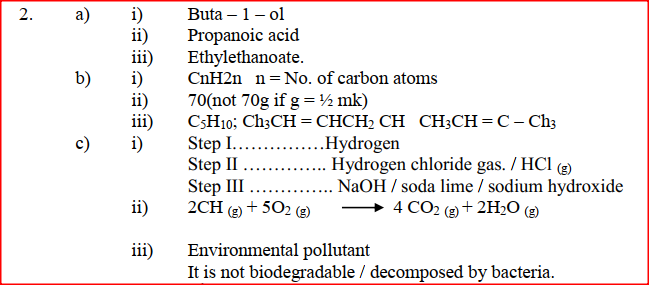
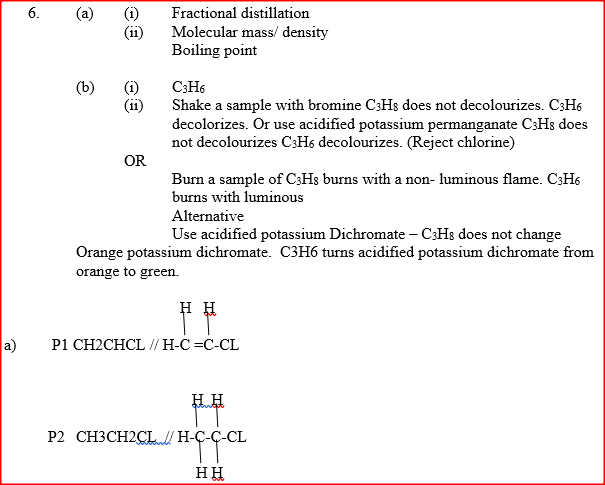
(a) Give the names of the following compounds
(b) Study the information in the table below and answer the questions that follow
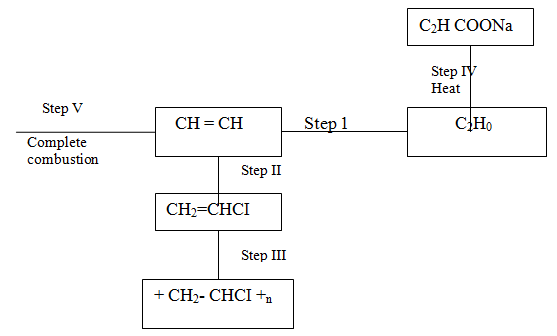
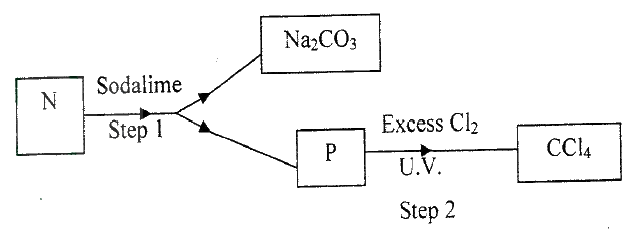
(i) Name the reagents used in:
Step I ……………………. Step II …………………….. Step III …………………….. (ii) Write an equation for the complete combustion of CH = CH (iii) Explain one disadvantage of the continued use of items made from the compound formed in step III
The relative formula mass of a hydrocarbon is 58. Draw and name two possible structures of the hydrocarbon (C=12.0; H=1.0)
A group of compounds called chlorofluorocarbons have a wide range of uses but they also have harmful effects on the environment.
State one: a) Use of chlorofluorocarbons b) Harmful effect of chlorofluorocarbons on the environments.
ANSWERS
a)Refrigeration
b) - They deplete the ozone layer. - They cause green house effect.
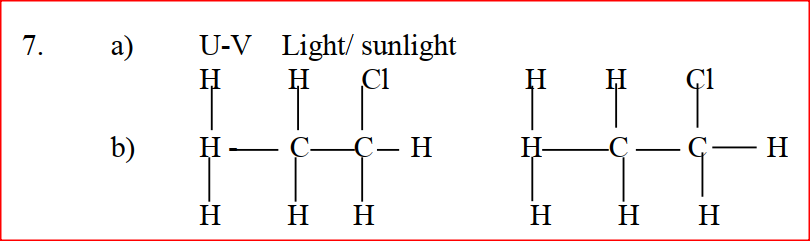
Name and draw the structure of the compound formed when methane reacts with excess chlorine in the presence of U.V light.
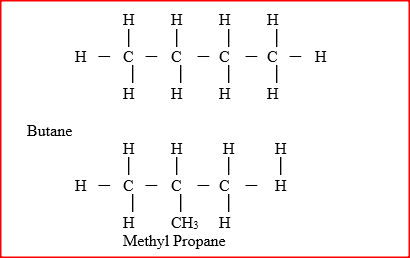
(a) What is meant by isomerism?
(b) Draw and name two isomers of butane.
ANSWERS
Give the name and draw the structural formula of the compound formed when one mole of ethane reacts with one mole of chlorine gas.
a) Crude oil is a source of many compounds that contain carbon and hydrogen only.
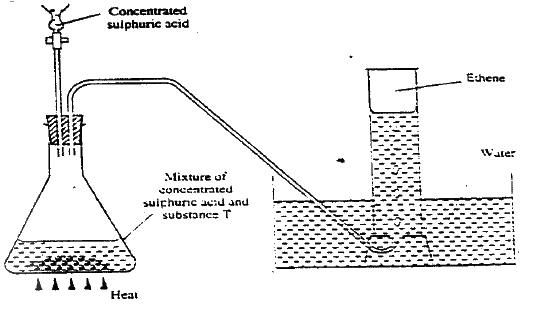
(i) Name the processes used to separate the components of crude oil (ii) On what two physical properties of the above components does the separation depend? b) Under certain conditions, hexane can be converted to two products. The formula of one of the products is C3H3 (i) Write the formula of the other product (ii) Describe a simple chemical reaction to show the difference between the two products formed in (b) above. c) Ethane, C2H2 is another compound found in crude oil. One mole of ethane was reacted with one mole of hydrogen chloride gas and a product p1 and was formed. P1 was then reacted with excess hydrogen gas to form p2. Draw the structures p1 and p2. d) The set-up below was used to prepare and collect ethane gas. Study it and answer the questions that follow.
(i) Name the substance T
(ii) Give the property of ethane that allows it to be collected as shown in the set up. e) One of the reactions undergone by ethane is addition polymerization. Give the name of the polymer and one disadvantage of the polymer it forms. (2 marks) Name the polymer. Disadvantage of the polymer
Expected response
Pentane: It is non poler and will not react with sodium Hydroxide solution which is an ionic compound.
Name the organic compound formed when CH3CH2CH2CH2CH2OH is reacted with concentrate sulphuric acid at 170°C
answer
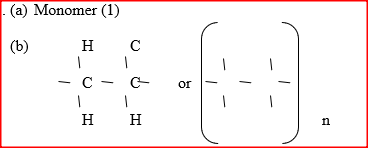
a) What is the name given to the smallest repeating unit of a polymer.
b) Draw the structure of the smallest repeating unit of a polyvinyl chloride
expected response
I, production of carbon dioxide or carbon is oxidized to its highest oxidation number/carbon dioxide cannot burn further or carbon dioxide cannot burn further or carbon monoxide can burn further
a) State the observation made when excess pentane is reacted with bromine gas
b) Name the compound formed in (a) above.
answers
But -2- ene undergoes hydrogenation according to the equation given below
CH3CH = CHCH3 (g) + H2 (g) →CH3CH2CH2CH3 (g) (a) Name the product formed when but -2 – ene reacts with hydrogen gas (b) State one industrial use of hydrogenation
answers
a)Butane
b)Hardening of oils in the manufacture of margarine The reaction of propane with chlorine gas gave a compound of formula C3H7Cl. a) What condition is necessary for the above reaction to take place? (1mk) b) Draw two structural formulae of the compound C3H7Cl (2mks)
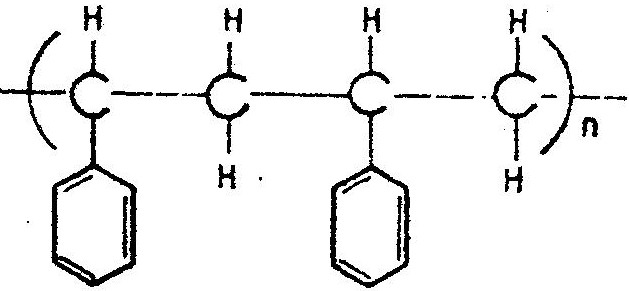
The structures below represents a portion of a polymer
(a) Give the name of the polymer (b) Give one industrial use of the polymer
answer
A compound C4H10O_ is oxidized by excess acidified potassium permanganate to form another compound C4H8O2. The same compound C4H10O reacts with potassium to produce hydrogen gas. a) Draw the structural formula and name the compound CaH10O (1mk) b) Write an equation for the reaction between potassium and compound C4H10O. |
Chemistry Topics
All
Archives
December 2024
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
|
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |
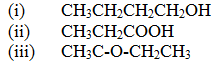








 RSS Feed
RSS Feed

