Differentiate between empirical and molecular formula (2mks)
Empirical formula show the simplest whole number ration in which atoms combine to for a compound while molecular formula shows actual number of each kind of atoms present in a molecule of a compound.
0 Comments
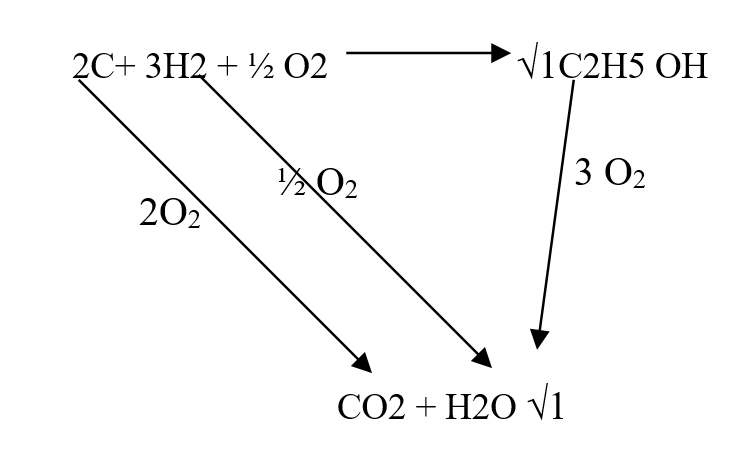
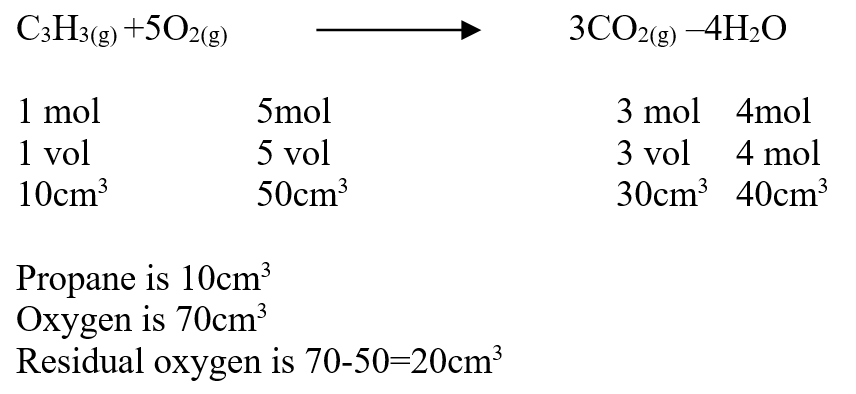
Use the information below to answer the questions that follow Ethanol is formed as shown below11/10/2021 Use the information below to answer the questions that follow Ethanol is formed as shown belowDraw the energy cycle diagram and for the formation and combustion of ethanol and calculate the heat of formation of ethanol (3mks)A volume of 80cm3 of a mixture of propane (C3H8) and oxygen were ignited in an experiment. The products were cooled and passed through an aqueous sodium hydroxide. The final volume was reduced by 30cm3a) Write the equation for the combustion of propane (1mk)b) Determine the volume of;i) The component of the original mixture (2mks)ii) Residual oxygen (1mk)a) State three differences between chemical and nuclear reactions. (3mks)
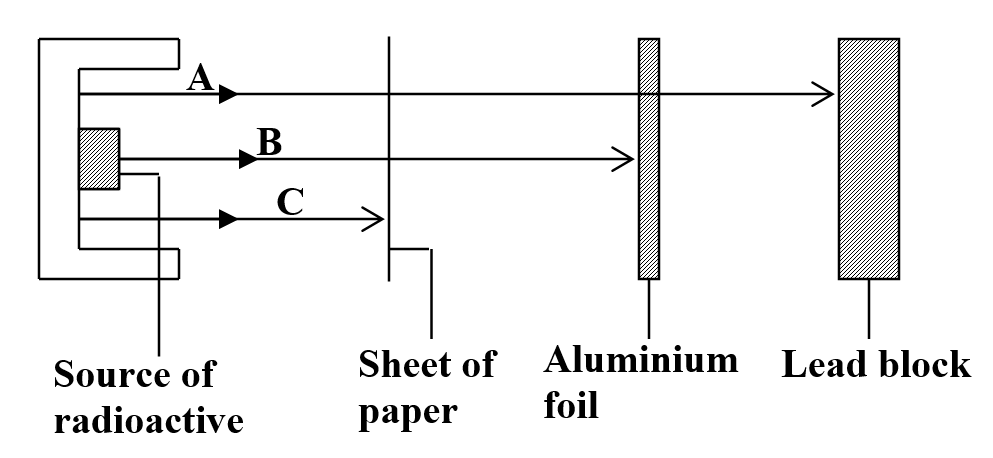
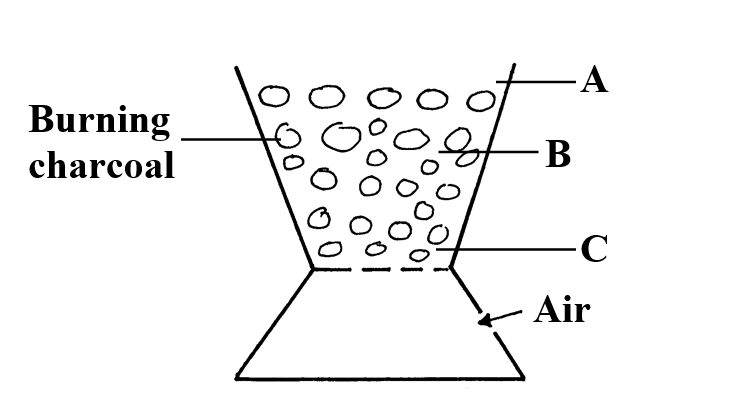
(any three 3mks) b) Study the figure below and answer the questions that followIdentify the radiations A, B and C (3mks)
RCOO – Na+ and RCH2OSO3 – Na+ represent two types of cleansing agentsa) Name the class of cleansing agent to which each belongs (1mk)b) Which one of the two cleansing agents is likely to pollute the environment. Explain. (2mks)RCH2OSO3-Na+ Because it contain long chains of alkylbenzene and sulphanate which is difficult to be broken by bacteria action a) Give a reason why ethanoic acid has a higher boiling point than ethanol which has the same number of Carbon atoms (1mk)
Ethanoic acid form hydrogen bond which are stronger than those in ethanol
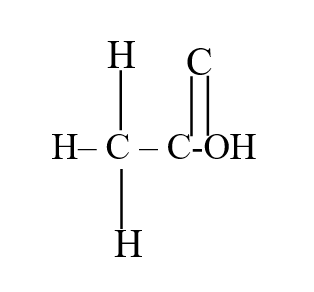
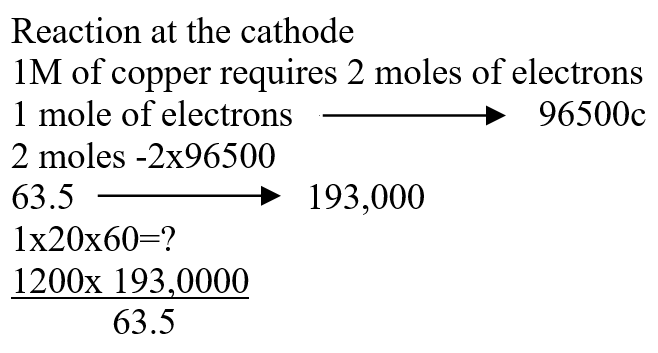
b) Draw the structural formula of ethanoic acid (1mk)a) Name one chief ore of copper and give its formula (2mks)Copper pyrites -CuFeS2 (any one 1mk) Cuprite – Cu2O2 Calculate the mass of copper that would be deposited on the cathode when a steady current of one ampere flows for 20 minutes through copper (II) sulphate solution (Cu = 63.5; Faraday Constance = 96500Cmol-1) (3mks)The cell convention for an electrochemical cell is shown belowa) Name two substances that can be used as electrolytes in the above cell (2mks)
Zinc sulphate or any other solution with zinc ions
b) Which of the electrodes is the anode? (2mks)
Lead electrode
Define oxidation and reduction in terms of electrons
Oxidation is the loss of electrons while reduction is gain electrons
Calculate the oxidation number of Chromium in Cr2O2- (1mk)
x * 2+(-2)=-2
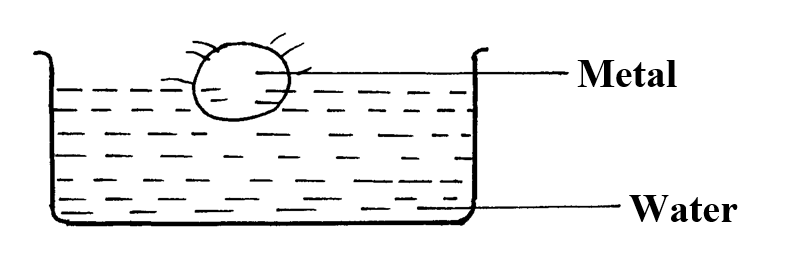
a) The solubility of the salt (2mks)b) The percentage of the salt in the saturated solution (1mk)The following diagram represents a charcoal burner. Study it and answer the questions that follow10/10/2021 Write the equations for the reaction at; (3mks)ABCi) Which metal is used above (1mk)
Potassium
ii) Which gas was produced (1mk)
Hydrogen
iii) What will be the colour of phenolphthalein indicator in the resulting solution? (1mk)
Pink
Name one gas used together with oxygen in welding other than acetylene gas (1mk)
Hydrogen
State two other uses of the gas named above (2mks)
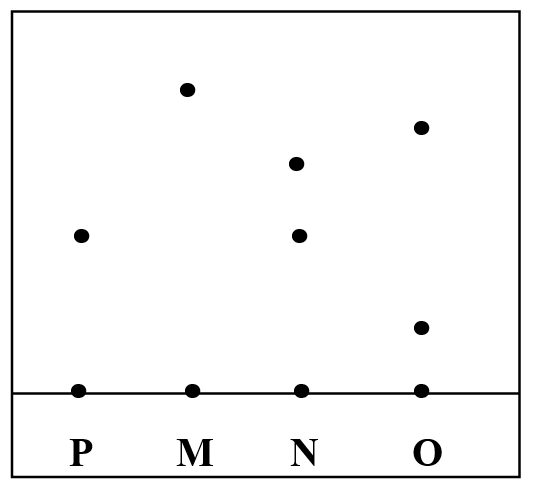
The result is shown below;i) Which ink was contaminated with substance P (1mk)
N
ii) Name the ink which was pure (1mk)
M
iii) Identify the other ink which was not pure (1mk)
O
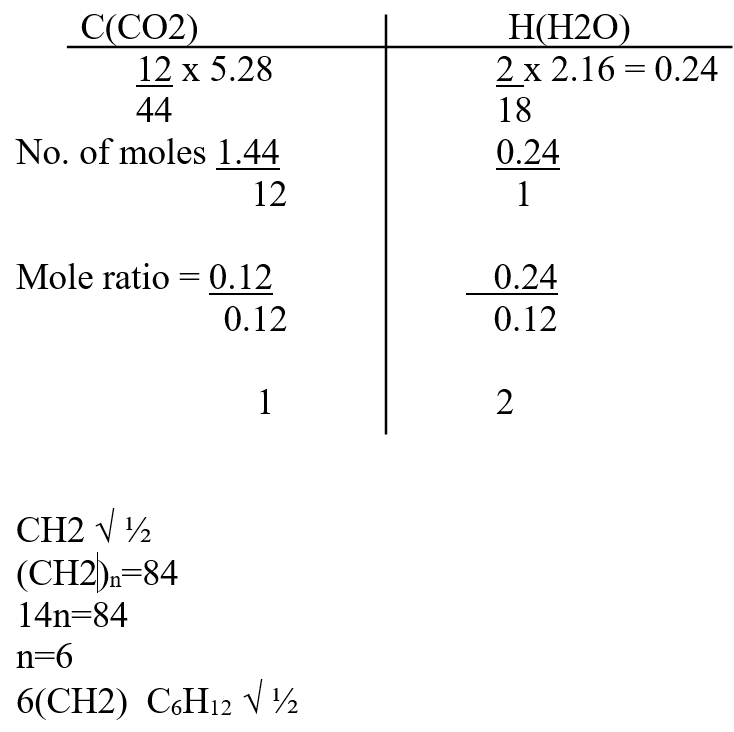
When a certain hydrocarbon burnt completely in excess oxygen 5.28g of Carbon (IV) oxide and 2.16g of water were formed. If the molecular mass of the hydrocarbon is 84, determine the molecular formula of the hydrocarbon Using dots (•) and cross (x) show the formation of Carbon (II) oxide gas (1mk)Name two types of bonds present in the molecule in ‘a’ above (2mks)
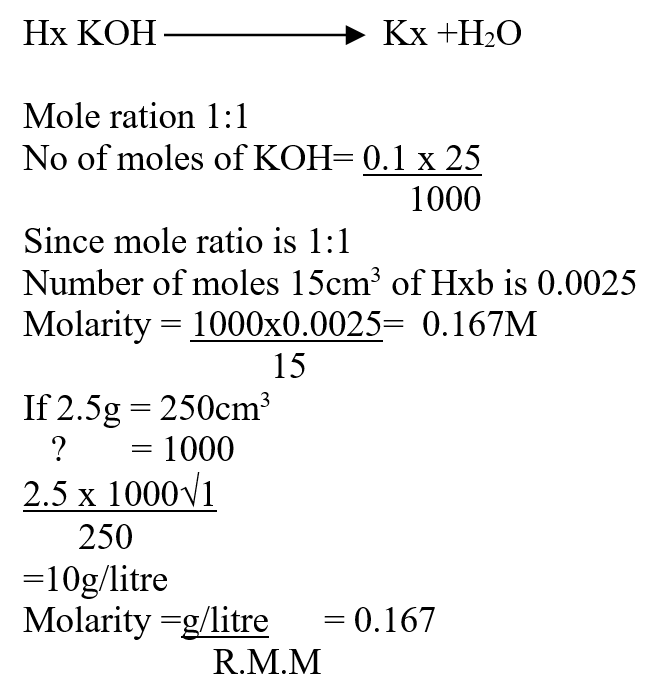
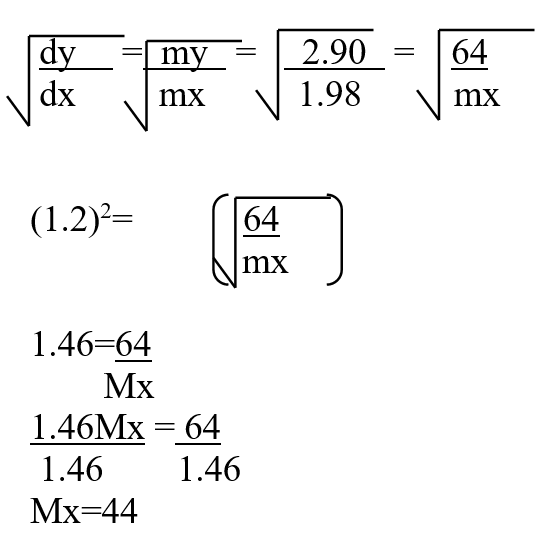
A mass of 2.5g of acid HX was dissolved in water and the resulting solution was diluted to a total of 250cm3, 15cm3 of the final solution was required to neutralize 25.0cm3 of 0.1M aqueous potassium hydroxide. Calculate the relative molecular mass of the acid (3mks)a) How do their rate of diffusion compare? (2mks)b) Determine the relative molecular mass of X given that the relative molecular mass of Y is 64 (1mk)a) Sugar crystals. (1mk)
b) Hydrated copper (II) sulphate solution (1mk)
c) What type of reaction has taken place above (1mk)
Alkaline earth metals are generally less reactive than alkali metals, explain.Alkaline earth metals loses two electrons while alkali metal lose one electron.
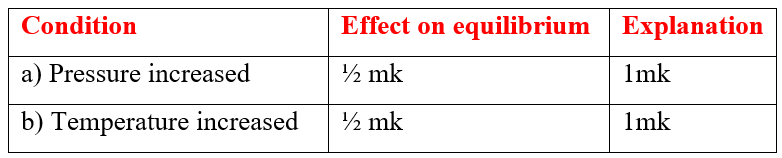
Ammonia gas is prepared by Haber process according to the equation belowN2(g) + 3H2(g) 2NH3(g) + HeatComplete the table below by stating the effect of equilibrium when the following conditions are applied. Give explanation in each caseSolutiona) Explain why sulphur is soluble in ethanol but hot in water (1mk)
b) Explain how a pure sample of sodium chloride can be obtained from a mixture of the two (1mk)
Explain why potassium is kept under paraffin while phosphorous is kept under waterPotassium does not react with paraffin but react with water while phosphorous react with paraffin but does not react with water
|
Chemistry Topics
All
Archives
December 2024
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
|
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |
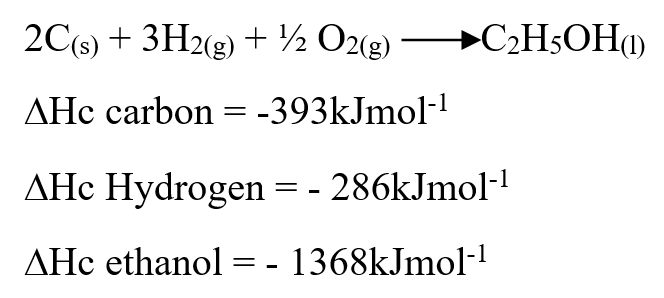
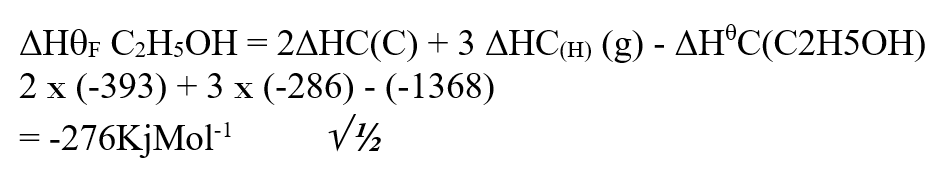
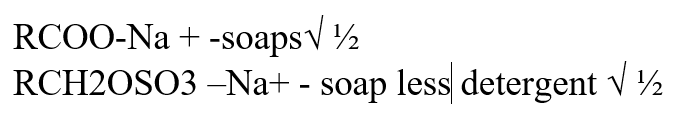
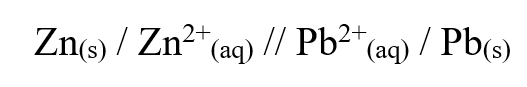
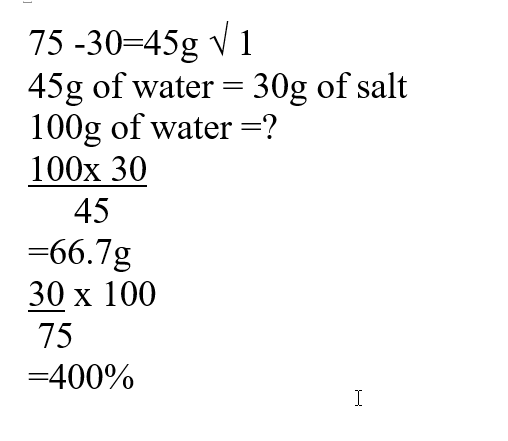

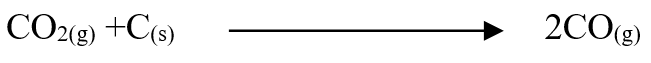



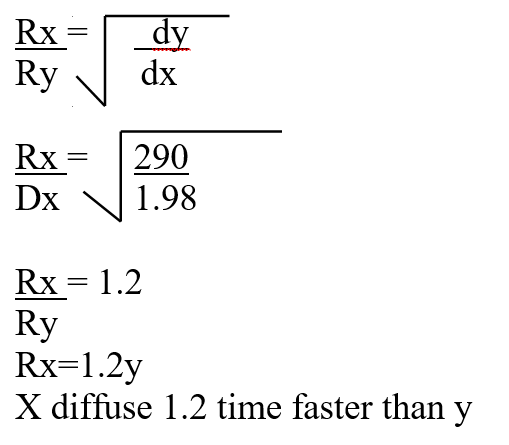


 RSS Feed
RSS Feed

