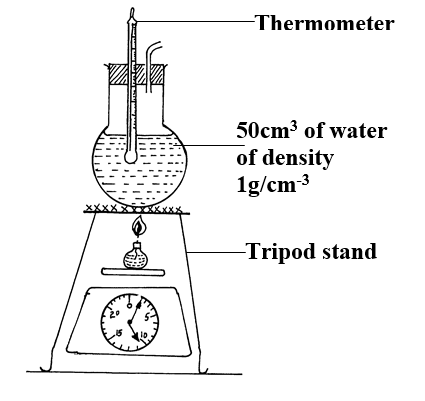
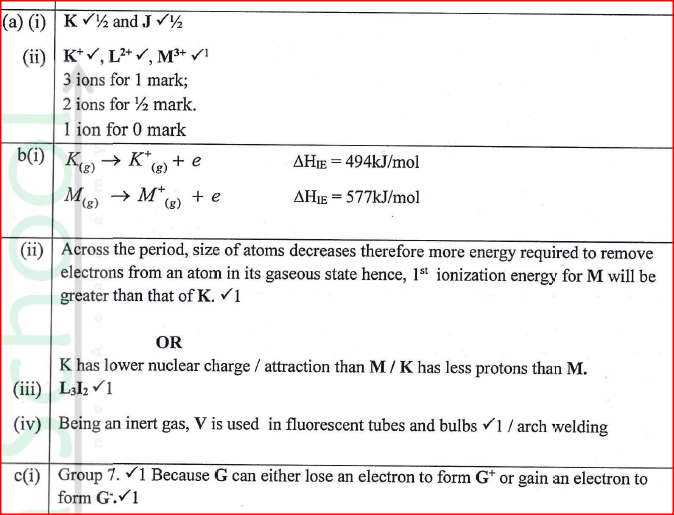
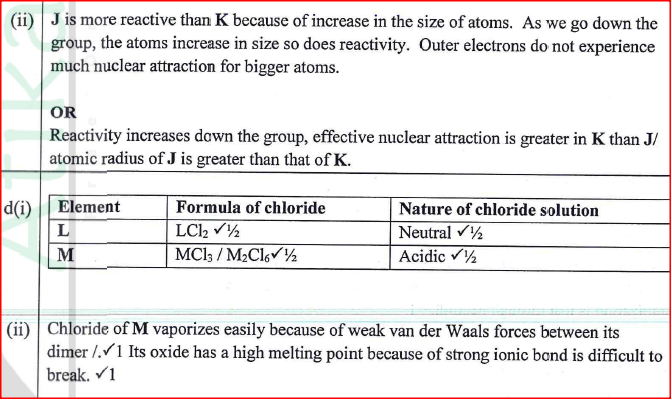
The following set – up was made in an experiment by a group of form four students. The readings of the balance before and after experiment were indicated in the diagram below. Given that the initial temperature of water was 26.7 degrees centigrade respectively. The specific heat capacity of water is 4200Jkg-1k-1
Determine:a) Temperature change that occurred (1mk)
b) Amount of ethanol used (1mk)
10.5 – 1.0 = 9.5g
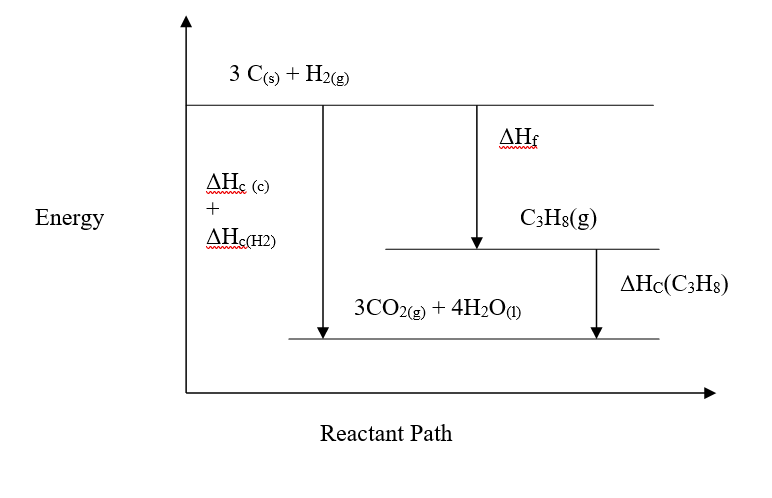
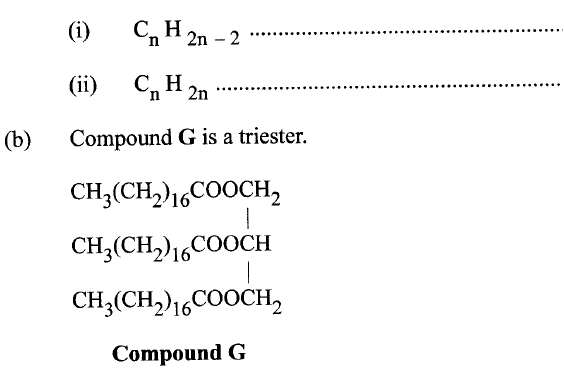
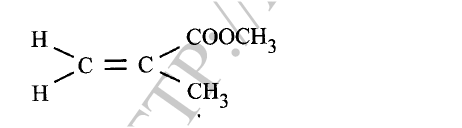
c) Moles of ethanol used (2mks)d) Amount of heat gained by water (2mks)e) Molar enthalpy of combustion of ethanol (2mks)f) Use the following thermochemical processes to answer the questions that follow;i) Draw an energy level diagram representing the formation and combustion processes of propane, carbon and hydrogen (2mks)
ii) Hence or otherwise, determine the heat of formation of propane (2mks)
0 Comments
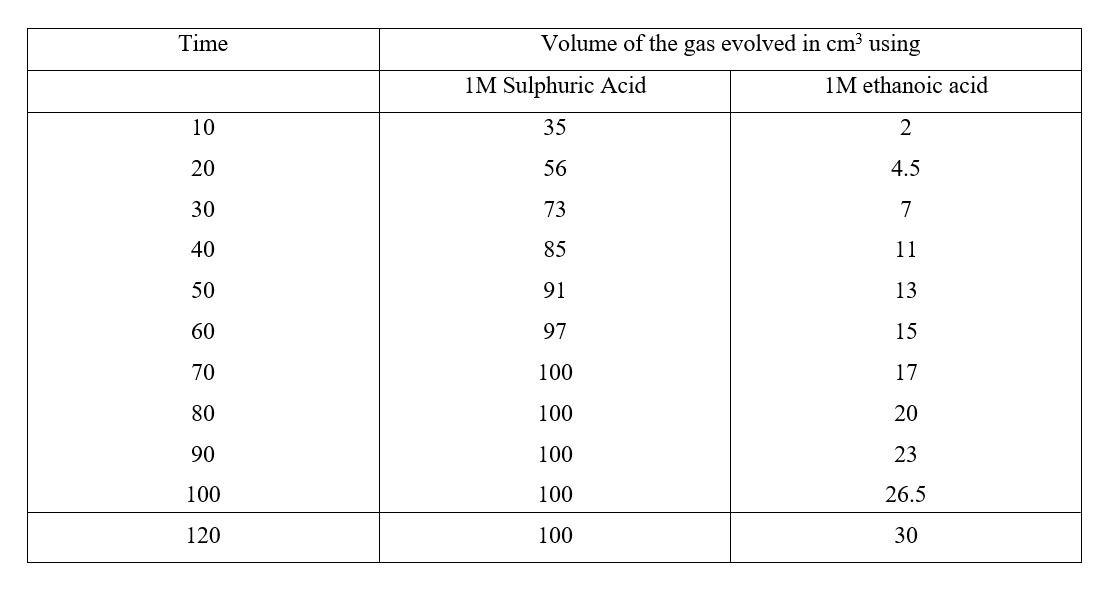
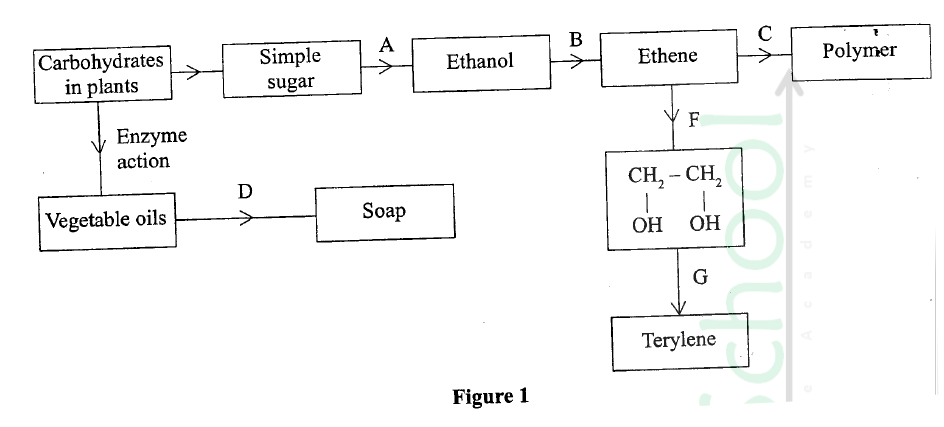
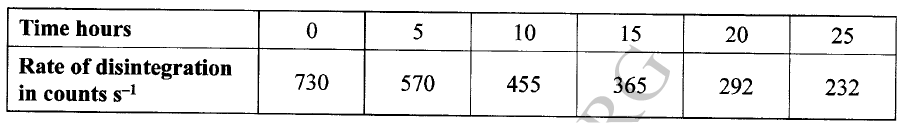
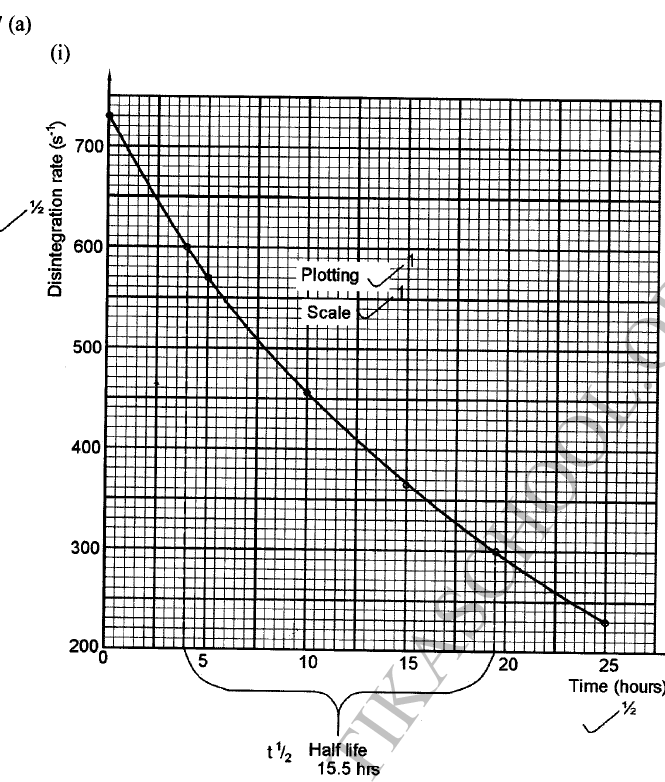
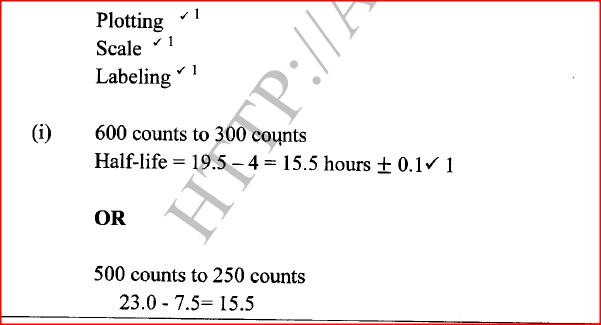
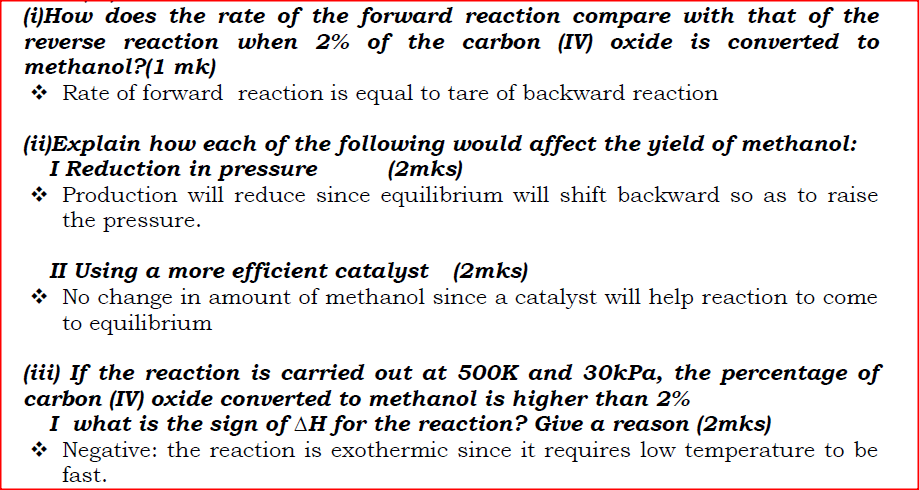
a) On the grid provided, plot on the same axis, the graph of volume of gas produced against time (4mks)
In the graph plotted ; Award marks as follows:
b) From the graph, determine the rate of reaction of both acids at 55 secondsi) 1M sulphuric (VI) acid (1mk)
Rate of 1M H2SO4 = 0.5 ± 0.05
ii) 1M ethanoic acid (1mk)
Rate of 1M ethanoic acid = 0.25 ± 0.01
c) The rate of reaction of H2SO4 is twice that of ethanoic acids. This is because H2SO4 is stronger while ethanoic acid is a weak acid.c) Explain the difference in the rate of evolution of the gas as determined in (b) above (2mks)
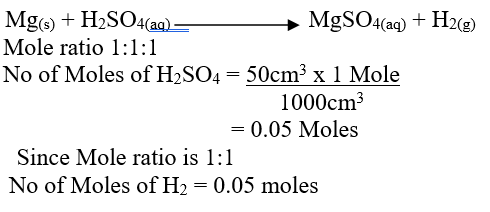
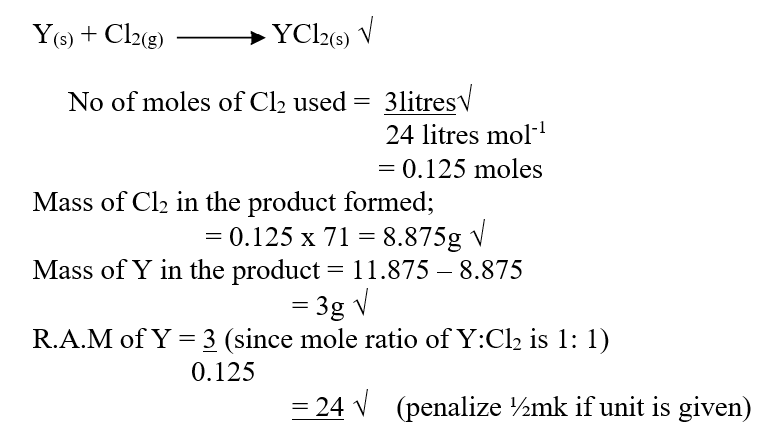
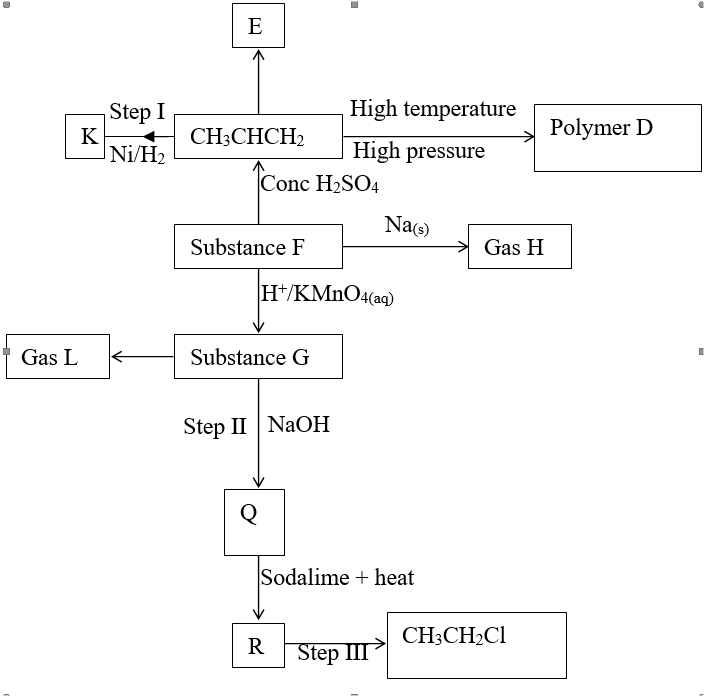
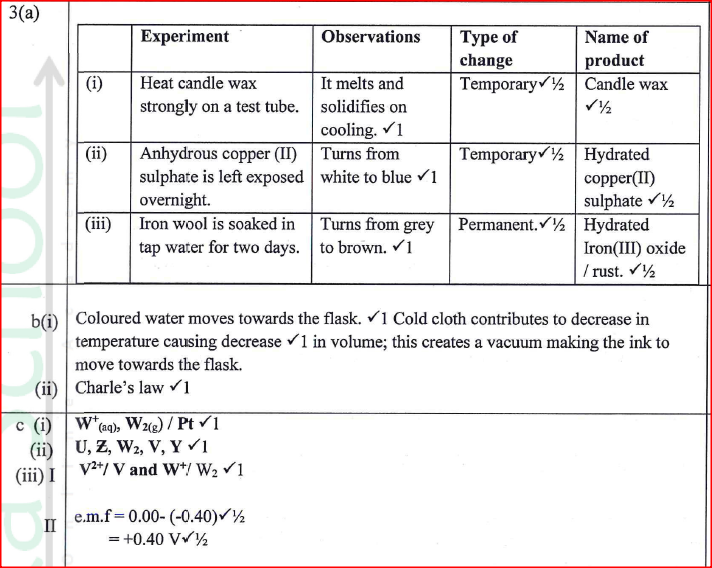
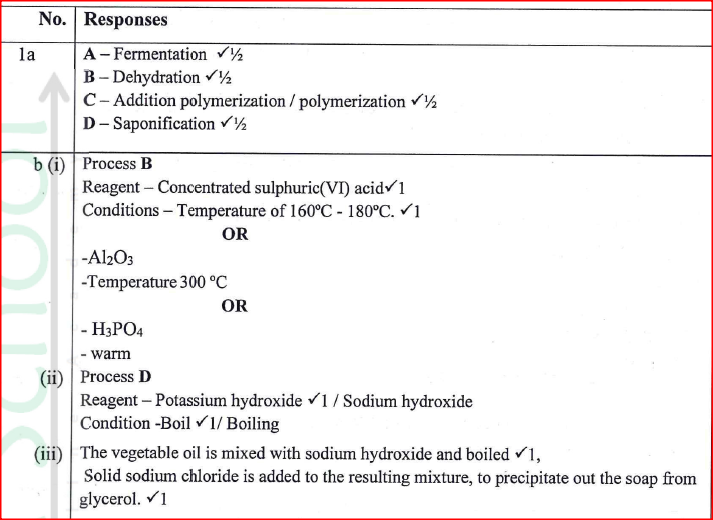
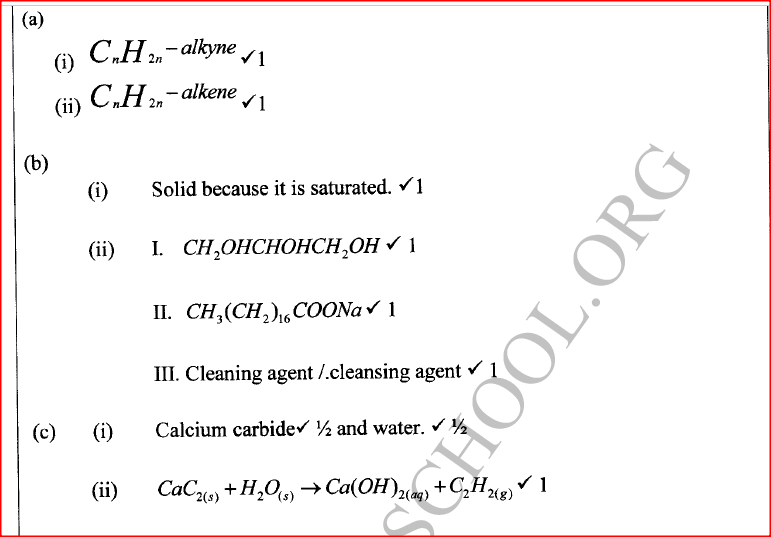
d) Calculate the number of moles of hydrogen gas produced when 10cm magnesium ribbon is completely reacted with 1M sulphuric (VI) acid. (Molar gas volume = 24dm3 at r.t.p) (2mks)e) What mass of magnesium had therefore reacted? (Mg = 24) (2mks)a) Name the substance the anode is made of (1mk)
Graphite
b) Explain your answer in (a) above (1mk)
Reject carbon
Graphite does not react with chlorine liberated at the anode
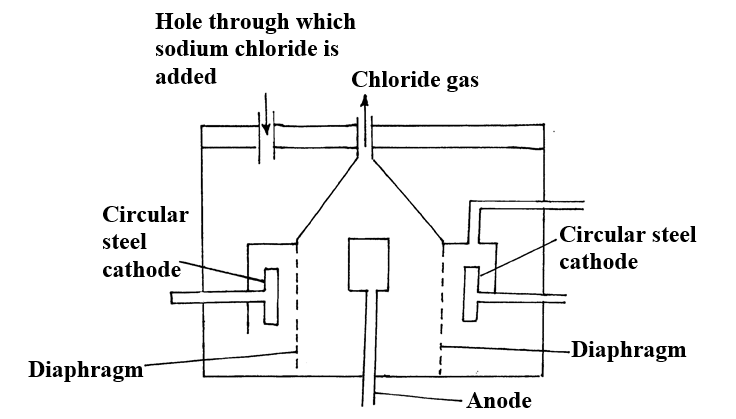
c) What is the role of the diaphragm in Down’s cell (1mk)
To prevent metal and chlorine gas from recombining
d) In Down’s cell for the manufacture of Sodium metal, Calcium chloride salt is added to lower the melting point from 8000C to 6000C. Explain why it is necessary to lower the melting point (1mk)
To reduce the cost of energy used to melt the sodium metal
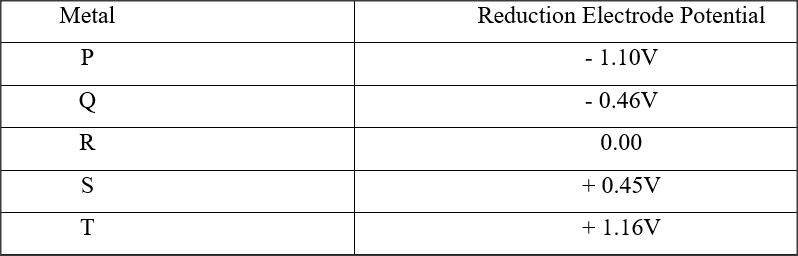
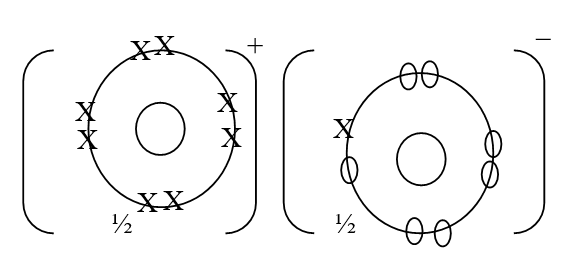
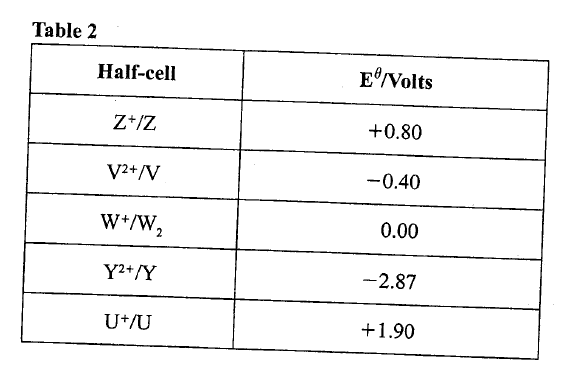
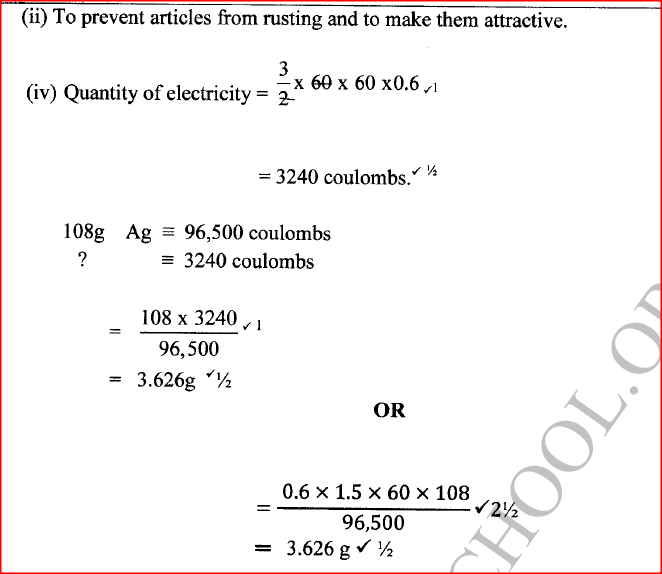
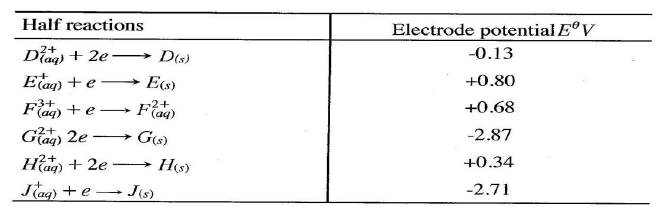
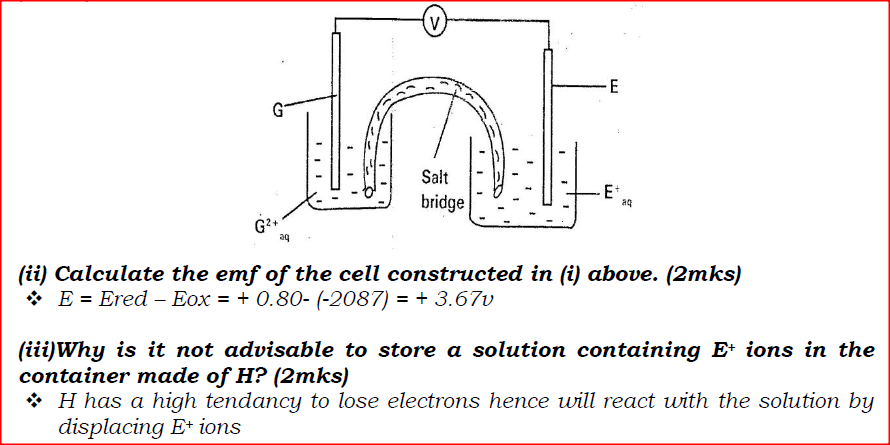
e) Calculate the mass of sodium metal produced if a current of 50 amperes is passed through the molten Sodium chloride for two (2) hours (Na = 23, F = 96500C) (2mks)f) Below is a list of potential differences obtained when metal P, Q, R, S and T are used in the following electrochemical cell
|
Chemistry Topics
All
Archives
December 2024
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
Can't find what you want? Use this Search box. |
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |
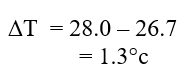
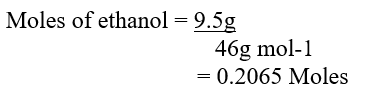
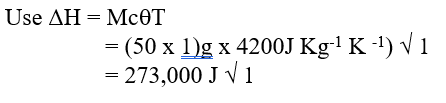

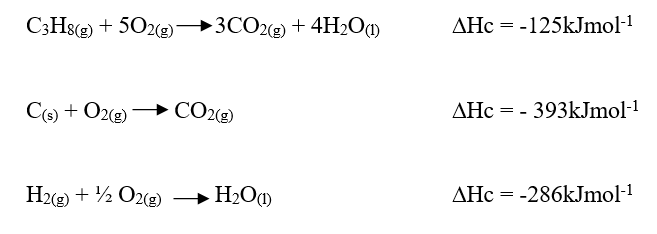
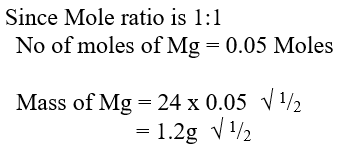
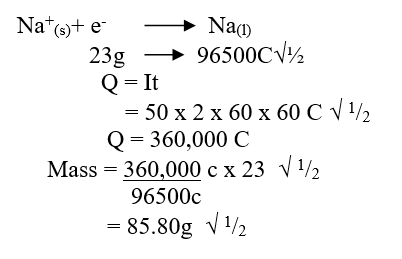





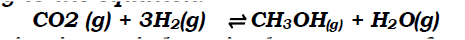

 RSS Feed
RSS Feed

