|
These are chemistry questions and answers categorized according to topics, papers i.e. Paper 1 and 2, Levels i.e. form 1 to form 4, kcse year the examination was done and section A or B
Select topic/category to open topical questions from that particular option provided. Chemistry Topics
0 Comments
a) State three differences between chemical and nuclear reactions. (3mks)
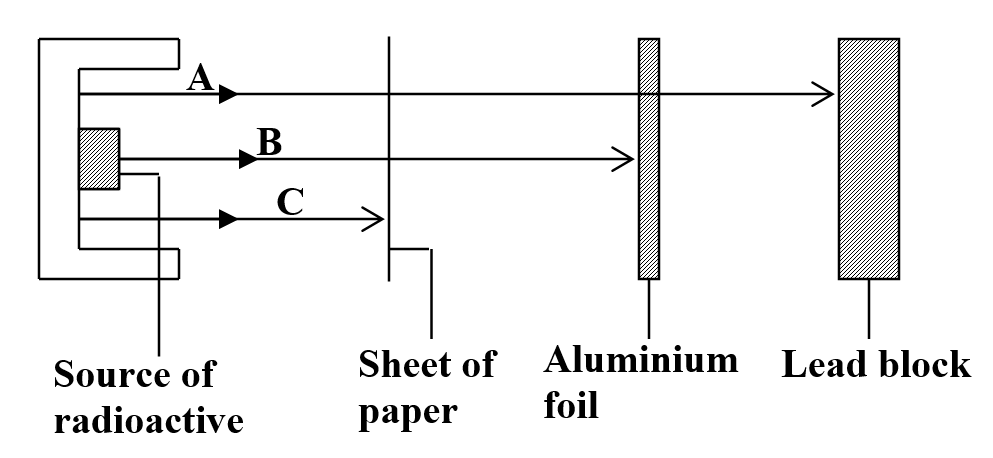
(any three 3mks) b) Study the figure below and answer the questions that followIdentify the radiations A, B and C (3mks)
(a) Give the symbols of the o charged particles emitted by a radioactive isotope.
mass number
atomic number:
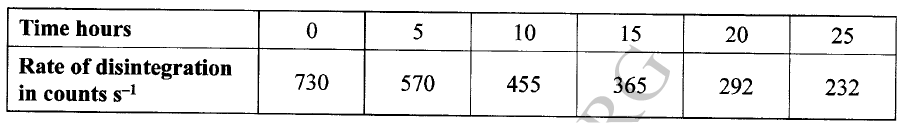
The decay rates of a sample of a radioisotope of bismuth at different time intervals is indicated in the following table.
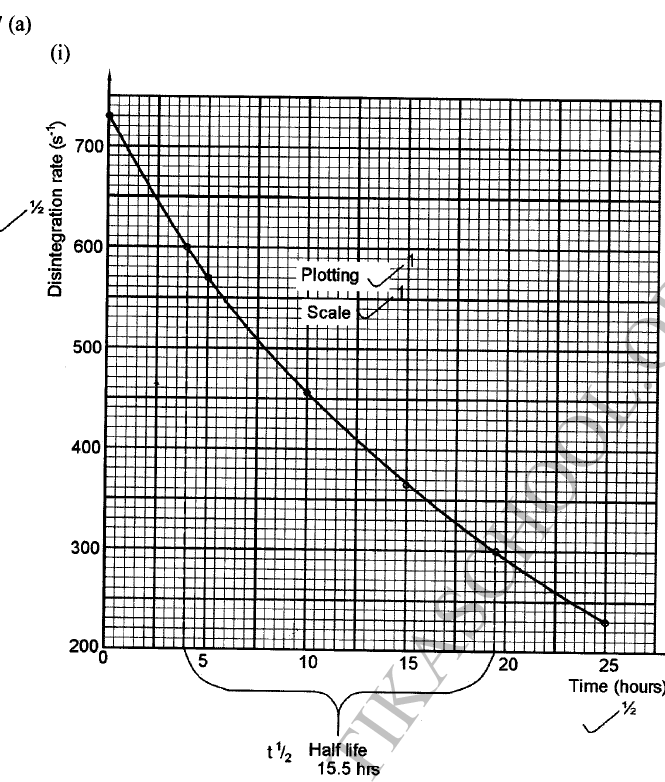
(a)(i) Draw a graph of disintegration rate against time.
(ii) Determine the half-life of bismuth. (iii) What would be the effect on the curve if half the amount of sample of bismuth were used. (b) Radioactivity has several applications. State one application of radioactivity in: (i) Medicine (ii) Agriculture (iii) Tracers (iv) Nuclear power station (c) State two dangers associated with radioactivity.
ANSWERS
(ii) It would have no effect on the curve as the quantity of bismuth does not affect half-life.
(b)(i)Applications in medicine Sterilizing surgical instruments. Destroying cancerous tissues during radiotherapy. Provide power to the heart pace setters. (ii) Applications in agriculture Monitor photosynthesis and other related processes. Preservation of foodstuffs, by exposing Micro-organisms to gamma rays. Rate of absorption of a fertilizer by the plant. (iii)Applications in Tracers Detecting leakages in underground water or oil pipes. (iv)Applications in Nuclear power stations. To generate electricity. (d) Dangers of radioactivity Long term exposure causes genetic mutation; Radioisotopes can be used as weapon of mass Destruction; Causes skin cancer; When tested causes environmental pollution.
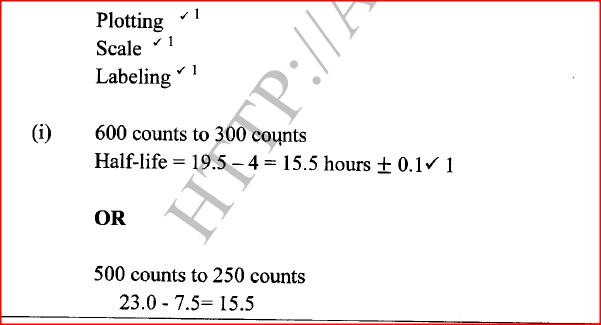
Calculate the values of X and Y in the following nuclear equation.
ANSWERS
a)Complete the nuclear equation below:
(c)Give one harmful effect of radioisotopes.
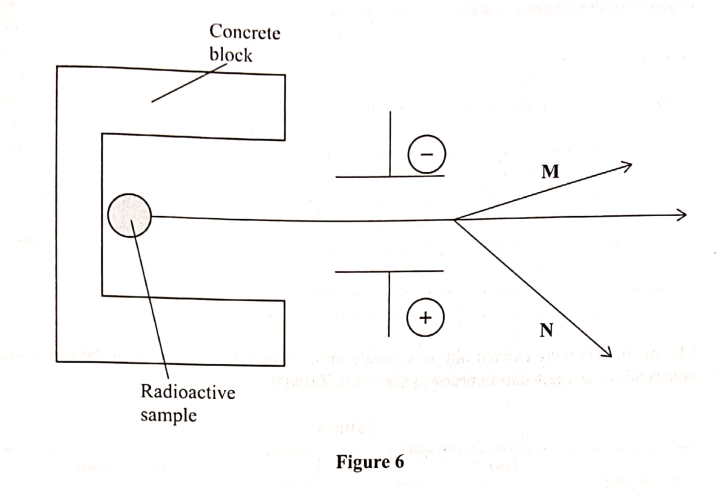
The diagram in Figure 6 Shows radiations emitted by a radioactive sample.
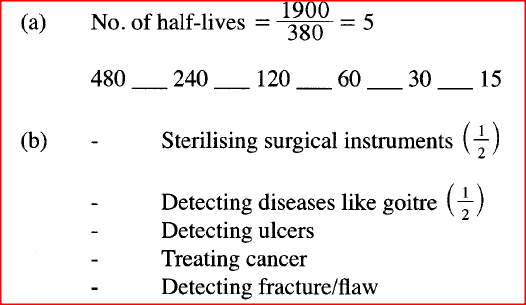
A radioactive substance weighing M kg took 1900 years for the original mass to reduce to 15 kg. Given that the half life of the radioactive substance is 380 years;
(a) Determine the original mass of the radioactive substance. (b) State two uses of radioactivity in medicine. .
(a) distinguish between a neutron and proton
(b) What is meant by a radioactive substance? (c) State two dangers associated with radioactive substance in the environment
(i) What is the atomic
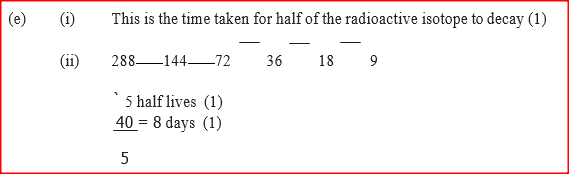
a. Mass of Y b. Number of Y (ii) What name is given to the type of reaction undergone by the isotopes of hydrogen? (e)(i) What is meant by half-life of a radioactive substance (ii) 288 g of a radioactive substance decayed to 9 g in 40 days. Determine the half-life of the radioactive substance
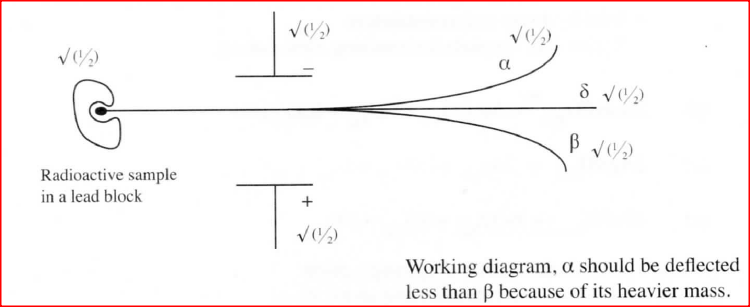
Draw a labeled diagram to illustrate how alpha, beta and gamma radiations can be distinguished from each other.
Study the nuclear reaction given below and answer the questions that follow
A radioactive isotope X2 decays by emitting two alpha (a) particles and one beta (β) to from 214 Bi 83 (a) What is the atomic number of X2? (b) After 112 days, 1/16 of the mass of X2 remained. Determine the half life of X2
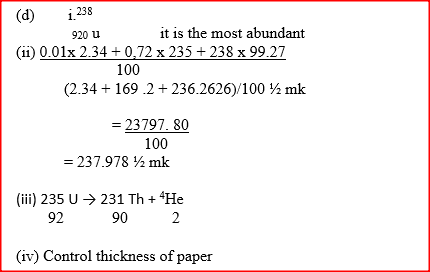
Expected Response
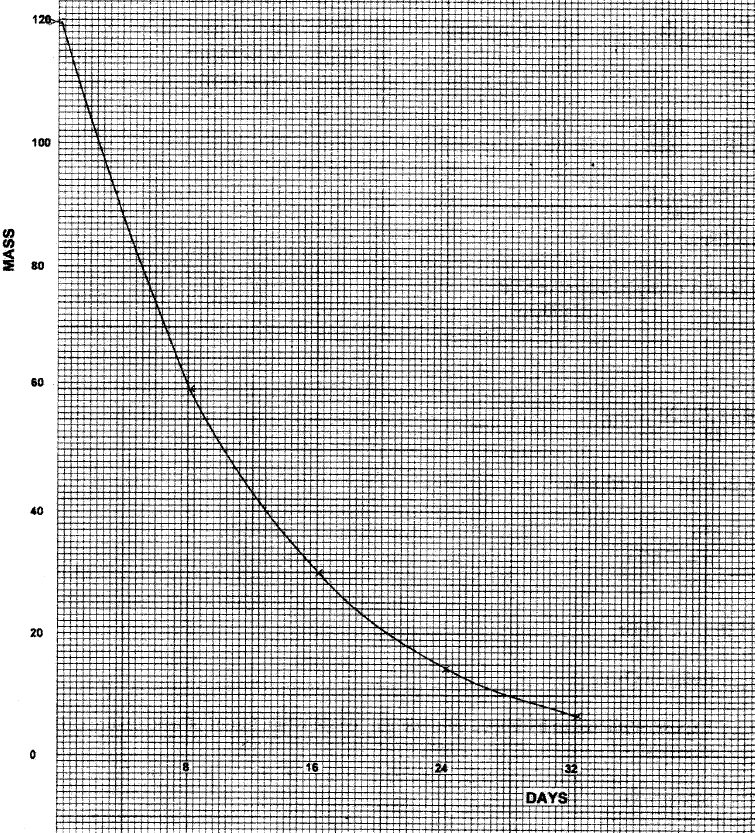
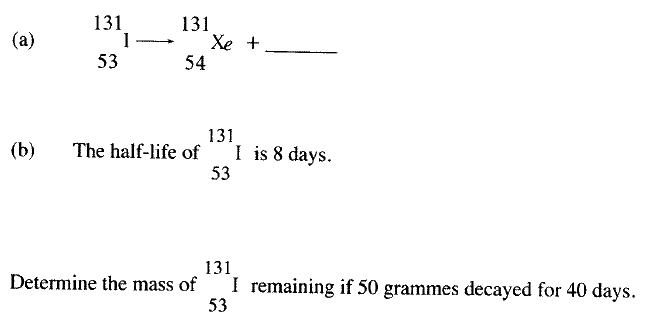
120g of iodine - 131 has a half life of 8 days and decays for 32 days. On the grid provided, plot a graph of the mass of iodine - 131 against time.
The graph below shows the mass of a radioactive isotope plotted against time
(a) Using the graph, determine the half – life of the isotope
(b) Calculate the mass of the isotope present after 32 days
Complete the nuclear equation below:
(c) Give one harmful effect of radioisotopes.
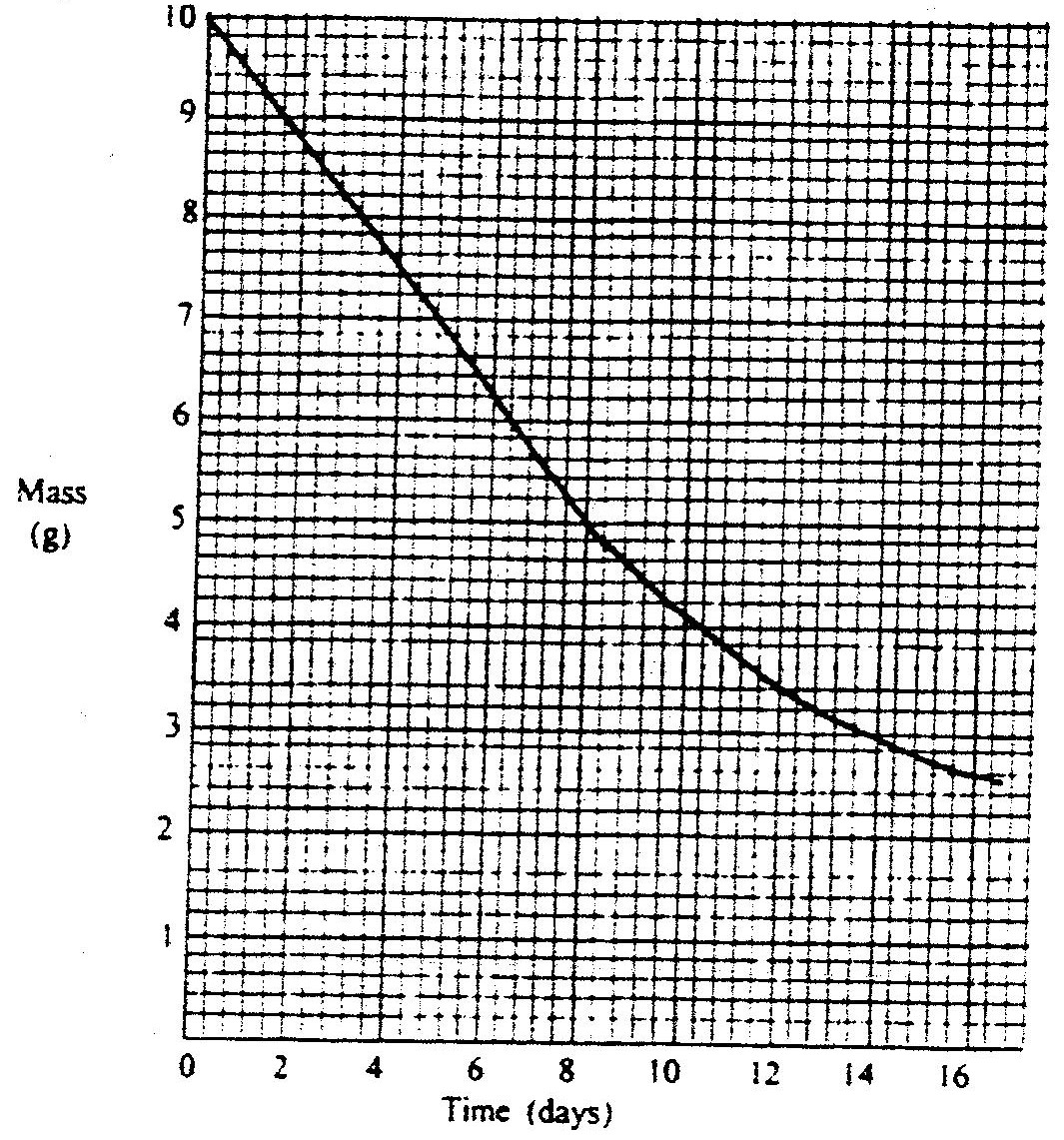
a) Write a nuclear equation for the decay process of carbon -14.
b) From the graph, determine the; i) Half-life of carbon -14; ii) Percentage of carbon -14 in a sample whose age is 1950 years.
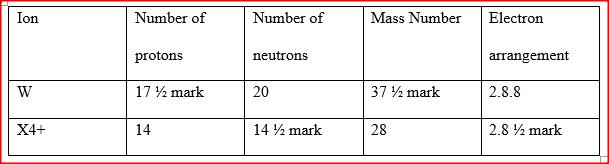
a) Study the table below and complete it. (W-1 and X4+ are not the actual symbols of the ions).
b) State the observation that would be made in the following tests to distinguish between:
i) Sodium and copper burning pieces of each in air. ii) Sodium and Magnesium by placing small pieces of each in cold water which contains two drops of phenolphalein. c) The atomic numbers of Na and Mg are 11 and 12 respectively. Which of the elements has a higher ionization energy? Explain. d) Naturally occurring uranium consists of three isotopes which are radioactive.
i) Which of these isotopes has the longest half-life? Give a reason
ii) Calculate the relative atomic mass of uranium
(iv)State one use of radioactive isotopes in the paper industry
ANSWERS
(b) (i) Sodium burns with a yellow flame & yellow white/ solid powder is formed while copper burn with a blue green flame & black powder/ silic is formed.
(ii) Sodium darts on the surface of water / rapid fast effervescence (fast production of bubbles; solution becomes pink immediately. Magnesium sinks in water/ slow (production of bubbles) effervescence/ solution becomes pink gradually. (c) Magnesium it has a higher nuclear charge which pulls outer electrons more strongly
An isotope of Uranium 234U, decays by emission of an alpha particle to thorium 92
a) Distinguish between nuclear fission and nuclear fusion.
b) Describe how solid wastes containing radioactive substances should be disposed of.
ANSWERS
(a) Nuclear fusion is where two light nuclei combine to give a heavy release of energy while nuclear fusion is where a large nuclear splits into smaller nuclei with the release of enormous amount of energy.
(b) Wrap with aluminium or lead foil and bury them deep underground
M grammes of a radioactive isotope decayed to 5 grammes in 100 days.
The Half – lift off the isotope is 25 days. a) What is meant by half – life? b) Calculate the initial mass of M of the radioactive isotope.
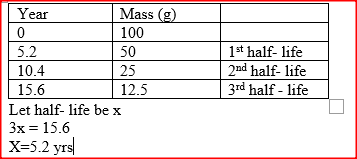
100 g of a radioactive substance was reduced to 12.5 g in 15.6 years. Calculate the half – life of the substance
State two factors which determine the stability of an isotope.
answers
|
Chemistry Topics
All
Archives
December 2024
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
|
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |

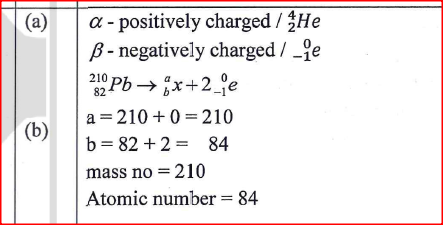
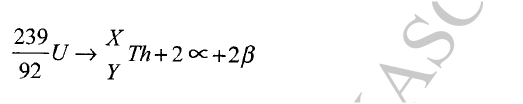

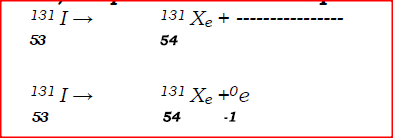
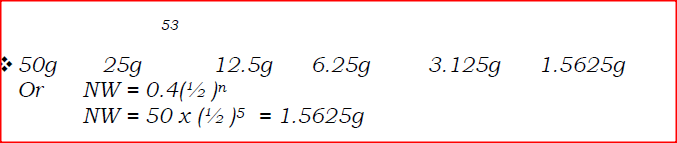


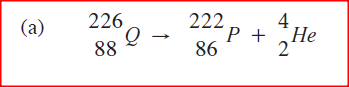

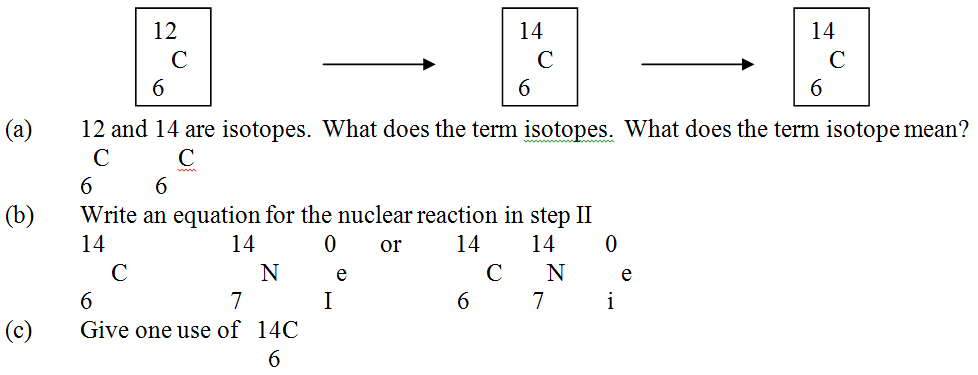
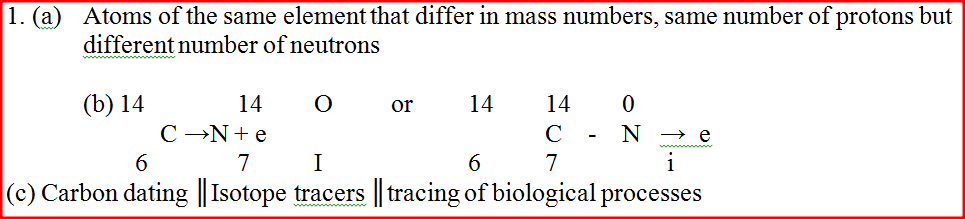
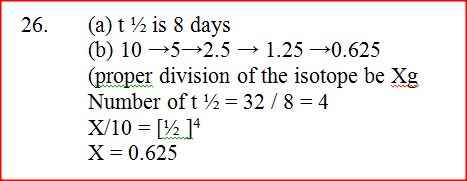


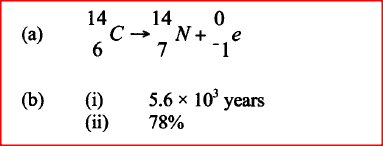
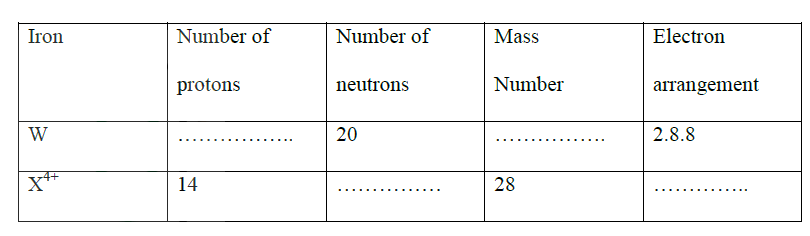
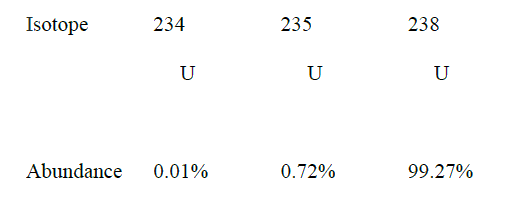
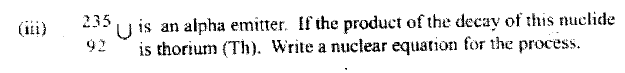
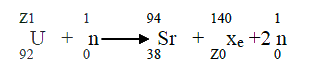

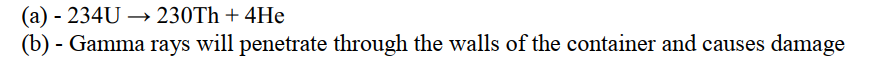

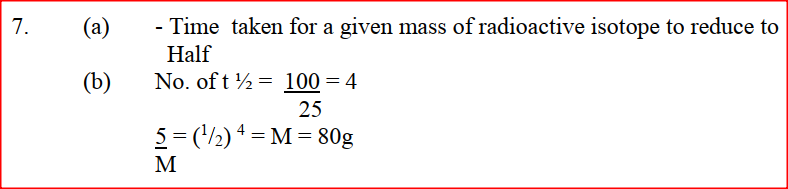

 RSS Feed
RSS Feed

