|
These are chemistry questions and answers categorized according to topics, papers i.e. Paper 1 and 2, Levels i.e. form 1 to form 4, kcse year the examination was done and section A or B
Select topic/category to open topical questions from that particular option provided. Chemistry Topics
0 Comments
Explain why a solution of sodium chloride Conducts electricity while that of sugar does not.
ANSWERS
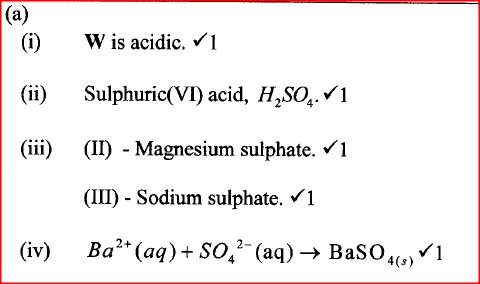
W is a colourless aqueous solution with the following properties:
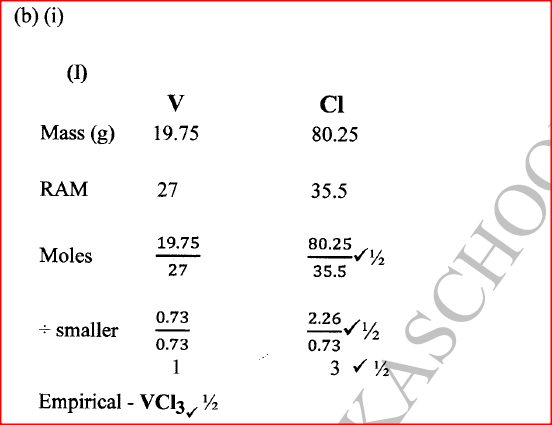
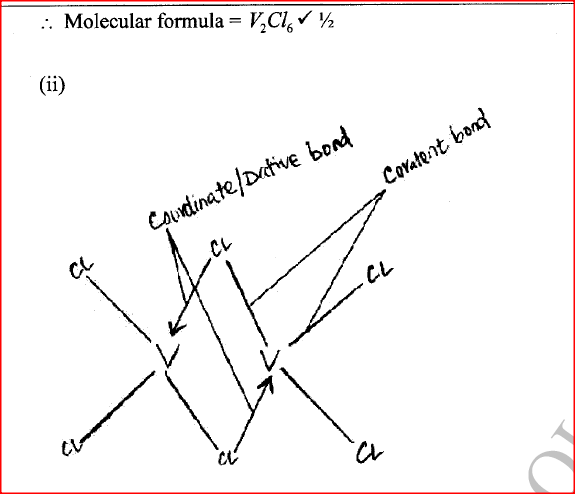
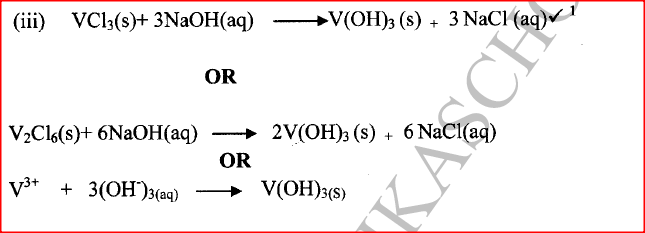
(ii) Give the identity of W. (iii) Name the colourless solution formed in (II) and (III). (iv) Write an ionic equation for the reaction indicated in (V). (b) Element V conducts electricity and melts at 933K. When chlorine gas is passed over heated V, it forms a vapour that solidifies on cooling. The solid chloride dissolves in water to form an acidic solution. The chloride vapour has a relative molecular mass of 267 and contains 19.75% of V. At a higher temperature, it dissociates to a compound of relative molecular mass 133.5. When aqueous sodium hydroxide is added to the aqueous solution of the chloride, a white precipitate is formed which dissolves in excess alkali. (V = 27.0 ; CI = 35.5) (i) Determine the:
(iii) Write an equation for the reaction that form a white precipitate with sodium hydroxide.
In terms of structure and bonding, explain why graphite is used as a lubricant in machines.
ANSWERS
The atomic numbers of some elements P, Q, R and S are 6, 8, 12 and 17 respectively.
(a) Draw the dot (•) and cross (X) diagrams for the compounds formed when: (i) R and Q react (ii) P and S react. (b) Explain why the melting point of the compound formed by P and S is lower than that formed by R and Q.
Table 1 shows the atomic numbers and the first ionisation energies of three elements. The letters are not actual symbols of the elements. Use it to answer the questions that follow.
(a) Explain the trend in first ionisation energy from A to C.
(b) Write the electronic configuration for the ion of C.
ANSWERS
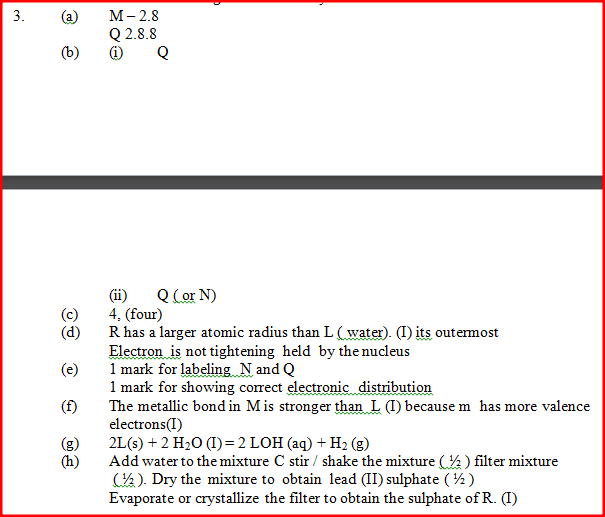
(a) Ionisation energy decreases down the group 1 elements.
This is because atomic radii increases from A to C (down the group) /outermost electron is far from nucleus hence requires less energy to be lost during reaction. (b)Electron configuration of ion of C- 2.8.8
(a) Write an equation to show the effect of heat on the nitrate of:
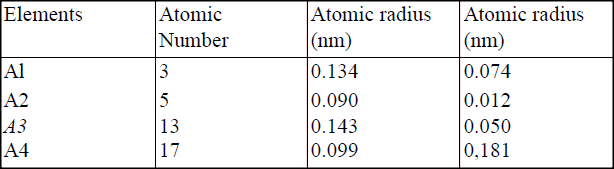
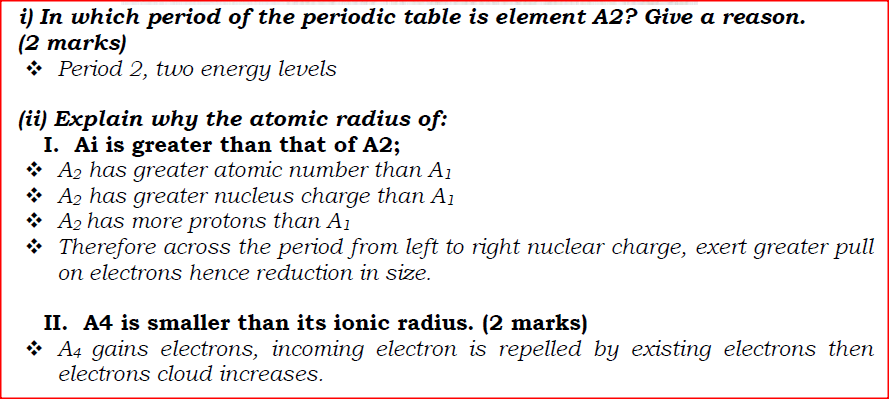
(i) Potassium (ii) Silver (b) The table below gives information about elements Ai, A2, A3, and A4 i) In which period of the periodic table is element A2? Give a reason.
(ii) Explain why the atomic radius of:
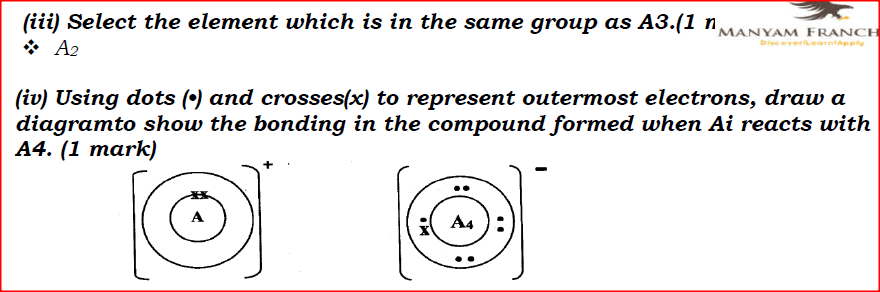
I. Ai is greater than that of A2; II. A4 is smaller than its ionic radius. (iii) Select the element which is in the same group as A3. (iv) Using dots (•) and crosses(x) to represent outermost electrons, draw a diagramto show the bonding in the compound formed when Ai reacts with A4.
Use the information in the table below to answer the questions that follow. The letters do not represent the actual symbols of the elements.
(a) Give reasons why the melting point of:
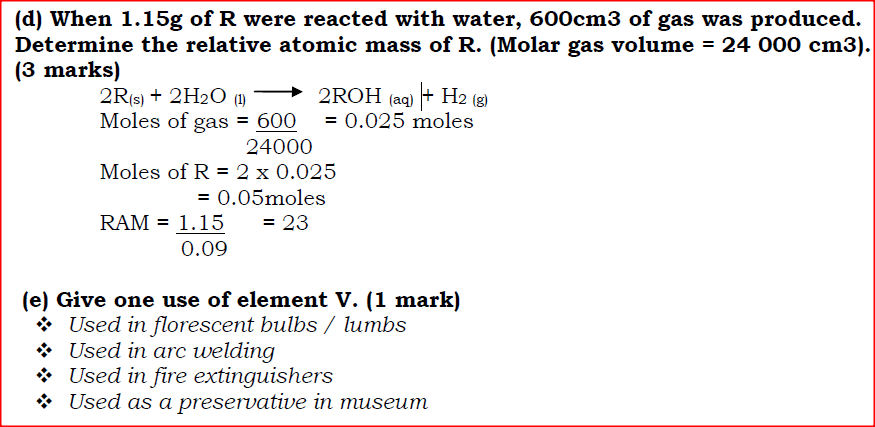
(i) S is higher than that of R; (ii) V is lower than that of U. (b) How does the reactivity of W with Chlorine compare with that of R with chlorine? Explain. (c) Write an equation for the reaction between T and excess oxygen. (d) When 1.15g of R were reacted with water, 600cm3 of gas was produced. Determine the relative atomic mass of R. (Molar gas volume = 24 000 cm3). (e) Give one use of element V.
Aluminium is both malleable and ductile.
(a)What is meant by? (i) Malleable: (ii)Ductile (b)State One use of aluminium based on: (i)malleability (ii)ductility
ANSWERS
(a) (i) Can be hammered into sheets.
(ii)Can be drawn into wires. (b)(i) Making of sufurias/ motor vehicle parts/ aeroplane parts,window / door flames, cups, plates, packaging materials, pans, making sheets/ roof. (ii)electricity cables/ wires.
(a) Name the method that can be used to obtain pure iron (III) chloride from a mixture of iron (III) chloride and sodium chloride.
(b) A student was provided with a mixture of sunflower flour, common salt and a red dye. The characteristics of the three substances in the mixture are given in the table below.
The student was provided with ethanol and any other materials needed.
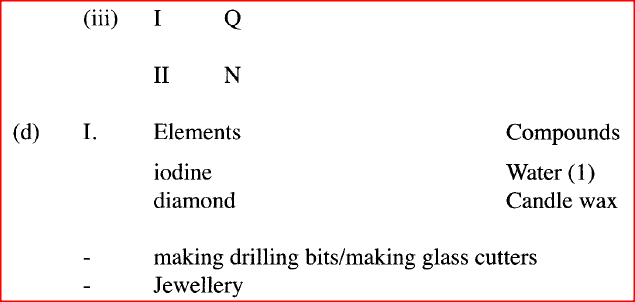
Described how the student can separate the mixture into its three components c) The diagram below show part of a periodic table. The letters do no represent the actual symbols of elements. Use the diagram to answer the questions that follow.
i) Explain why the oxidizing power of W is more than that of X
ii) How do the melting points of R and T compare? Explain iii) Sketch an element that could be used i) In weather ballons ii) For making a cooking pot d i) Classify the substances water, iodine, diamond and candle wax into elements and compounds
ii) Give one use of diamond
ANSWERS
(a)Sublimation
(b)Add ethanol to the mixture . Filter and evaporate filtrate to obtain red dye . Add water to the residue . Filter to obtain sunflower flour . Evaporate filtrate to obtain salt . OR Add H,O to mixture , filter , residue is sunflower , evaporate the water ; add ethanol to the residue filter . The filtrate is red dye. (3 marks) (c)(i) W accepts electrons more readily than X. W has small atomic radius/ W has less energy levels than X/ W has less screening effect than X/ W has greater effective nuclear attraction than X. W is more electro negative than X. (ii) T has a lower melting point than R because it exists in simple molecular form with weak Van der Waals forces while R has strong metallic bonds.
A crystal of iodine, heated gently in a test tube gave off a purple vapour.
(a) Write the formula of the substance responsible for the purple vapour. (b) What type of bond is broken when the iodine crystal is heated gently? (c) State one use of iodine.
ANSWERS
(a)Formula of Iodine I2
(b)Weak Van der Waals (c)Antiseptic
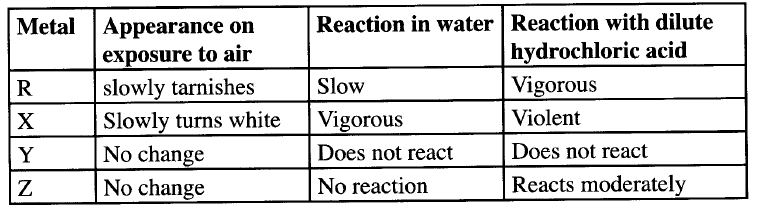
The table below shows behaviour of metals R, X, Y and Z. Study it and answer the questions that follow:
a) Arrange the metals in the order of reactivity starting with the most reactive
b) Name a metal which is likely to be i X ii Y
ANSWERS
(a) Reactivity series starting with the most reactive
X R Z Y (b) X could be potassium Y could be copper
The atomic number of an element, T is 15.
(a) Write the electronic configuration of the ion T (b) Write the formula of an oxide of T.
Table 1 shows the properties of two chlorides, D and E.
(a) State the type of bond present in:
Given that the atomic number of Y is 13 and that of Z is 9:
a)Write the electronic arrangement of Y and Z; b) Draw the dot (.) and cross(x) diagram for the compound formed by Y and Z
The table below shows the properties of substances K,L,M and N
Select the substances which are likely to be:
a) Copper metal b) Magnesium chloride
Expected Response
(a) Copper metal M
(b) Magnesium chloride K The melting point of phosphorous dichloride is – 910C. While that of magnesium chloride is 7150C.In terms of structure and bonding, explain the difference in their melting points.
Expected Response
In terms of structure and bonding, explain the following observations:
(a) The melting point of aluminum is higher than that of sodium (b) Melting point of chlorine is lower than that of sulphur
ANSWERS
(a) Aluminium has a stronger metallic bond because it has more delocalised electronsthan sodium.
(b) Sulphur has a ringed structure of S8 molecules whiles chlorine is diatomic. The forces in sulphur are stronger than chlorine.
The grid below is part of the periodic table. Use it to answer the questions that follow. (The letters are not the actual symbols of the elements)
a) Which is the most reactive non-metallic element shown in the table? Explain.
b) i) Write the formula of the compound formed when element A reacts with element B. ii) Name the bond type in the compound formed in b (i) above. c) i) What is the name given to the group of elements where C, G and H belong? ii) Write an equation for the reaction that occurs when C in gaseous form is passed through a solution containing ions of element H. d) The melting points of elements F and G are 1410°C and -101°C respectively. In terms of structure and bonding, explain why there is a large difference in the melting points of F and G. e) D forms two oxides. Write the formula of each of the two oxides. f) J is an element that belongs to the 3rd period of the periodic table and a member of the alkaline earth elements. Show the position of J in the grid.
Use the following information on substances S, T, V and hydrogen to answer the questions that follow:
(i) T displaces V from a solution containing V ions. (ii) Hydrogen reacts with the heated oxide of S but has no effect on heated oxide of V. (a) Arrange substances S, T, V and hydrogen in the order of increasing reactivity. (b) If T and V are divalent metals, write an ionic equation for the reaction in (i) above.
Compound Q is a solid with a giant ionic structure. In what form would the compound conduct an electric current
Expected Response
16. When dissolves in water or fused / molten state
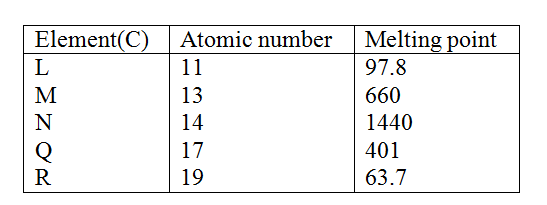
Study the information in the table below and answer the questions that follow. The letters do not represent the symbols of the elements.
a) Write the electrons arrangement for the atom formed by elements and M and Q
b) Select an element which is
d) Element R lodes its outermost electron more readily than I. Explain e) Using dots(.) and crosses (x) to represent outermost electrons show bonding in the compound formed elements N and Q. f) Explain why the melting point elements M is higher than that of element . g) Describe how a solid mixture of sulphate of R and lead sulphate can be separated into solid samples.
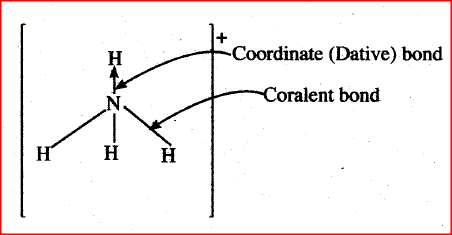
Ammonium ion has the following structure:
Label on the structure:
(a) covalent bond;
(b) coordinate (dative) bond.
(a) Other than their location in the atom, name two other differences between an electron and a proton.
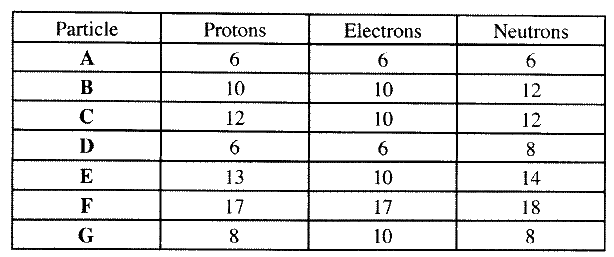
(b) the table below gives the number of electrons ,protons and neutrons in particles A,B, C , D, E, F and G
(i) Which particle is likely to be a halogen?
(ii) what is the mass number of E (iii) write the formula of the compound formed when E combines with G iv) Name the type of bond formed in (iii) above. (v) How does the radii of C and E compare ? Give reason. (vi) Draw a dot (.) and cross(x) diagram for the compound formed between (vii) Why would particle B not react with particle D?
ANSWERS
(a)- electron has I mass while proton has mass of one mass unit. 1840
- proton is positively charged while electron is negatively charged.
(iv)Ionic bond or electrostatic
(v)E has a smaller atomic radius than C E has more protons than C : nuclear attraction stronger.
(vii)Particle B is inert with a stable electronic configuration will not react.
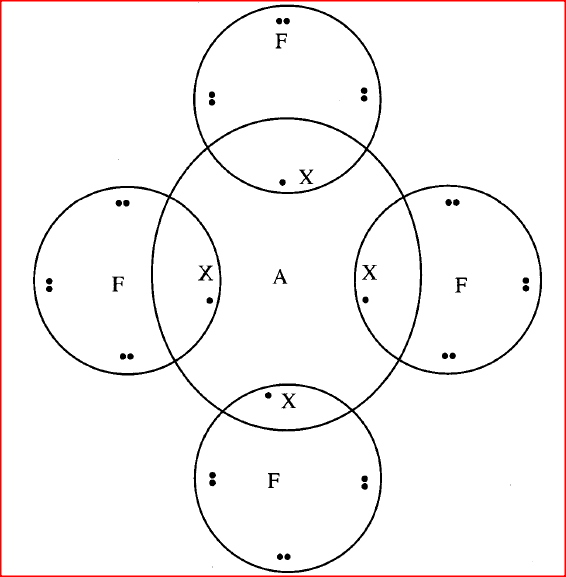
The diagram below shows the bonding between aluminium chloride and ammonia.
(a) Name the types of bonds that exist in the molecule.
(b) How many electrons are used for bonding in the molecule? |
Chemistry Topics
All
Archives
December 2024
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
|
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |
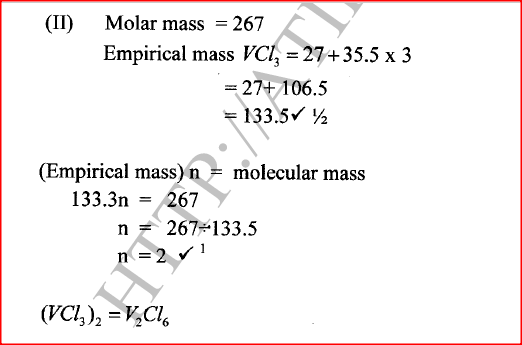



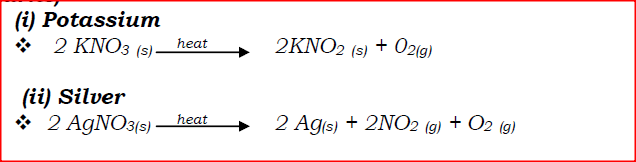

















 RSS Feed
RSS Feed

