|
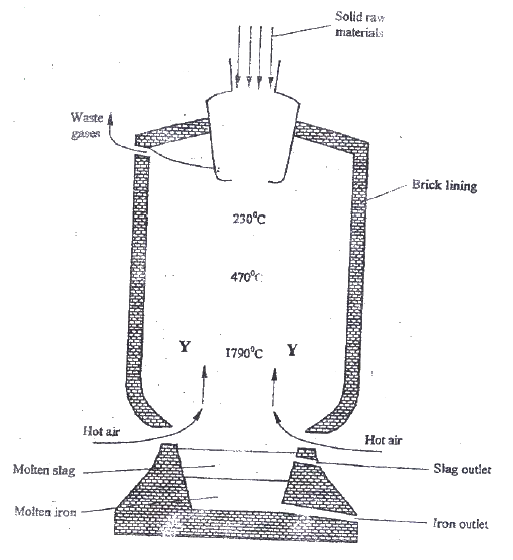
Iron is obtained from haematite using a blast furnace shown if figure 5 below.
a) Four raw materials are required for the production of iron. Three of these are iron oxide, hot air and limestone
Give the name of the fourth raw material b) Write an equation for the reaction in which carbon (IV) oxide is converted into carbon (II) oxide. c) Explain why the temperature in the region marked Y is higher than that of the incoming hot air. d) State one physical property of molten slag other than density that allows it to be separated from molten iron as shown in figure 5. e) One of the components of the waste gases is Nitrogen (IV) oxide describe the adverse effects it has on the environment. f) Iron from the blast furnace contains about 5% carbon i) Describe how the carbon content is reduced. ii) Why is it necessary to reduce the carbon content?
ANSWERS
(a) Coke/ coal/ Charcoal/ Carbon
(b) C(s) + CO2 (g) → 2 CO(g) (c) The reaction between coke/ coal and the hot air is highly exothermic (d) Slog is immiscible with molten iron (e) Nitrogen (iv) oxide gas forms acid rain. Which corrodes metallic materials and destroys vegetation the environment. (f) (i) By passing/ blowing oxygen into molten iron which converts carbon into carbon (iv) Oxide (ii) To increase the tensile strength/ making the iron less brittle/ making it more malleable / making it more ductile. Related Chemistry Questions and Answers on Metals Form 4 Level
0 Comments
Leave a Reply. |
Chemistry Topics
All
Archives
December 2024
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
|
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |

 RSS Feed
RSS Feed

