|
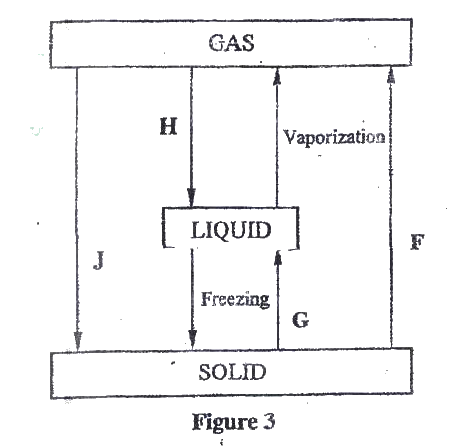
(a)Figure 3 show the changes that take place between states of matter. Some of them have been identified and others labelled.
i) Give the names of the processes
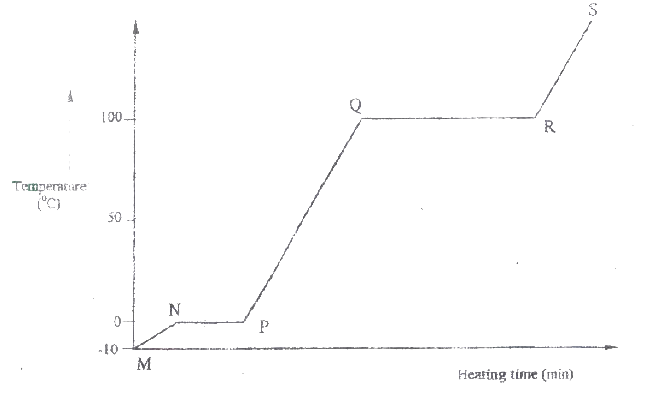
I H II G ii) Name one substance that can undergo process F when left in an open container in the laboratory. iii) The process J is called deposition. Using water as an example, write an equation that represents the process of deposition. b) Figure 4 shows the beating curve for water.
i) Give the names of the intermolecular forces of attraction in the segments;
I MN II RS ii) The heats of fusion and vaporization of water are 334.4 Jg-1 and 1159.4 Jg-1 respectively. I Explain why there is a big difference between the two. II How is the difference reflected in the curve? c) Coal, oil and natural gas are major sources of energy. They are known as fossil fuels. Hydrogen is also a source of energy. i) State and explain two reasons why hydrogen is a very attractive fuel compared to fossils. ii) State one disadvantage of using hydrogen fuel instead of fossil fuels.
ANSWERS
(a)
(i) I. Condensation II. Melting (ii) Iodine, Benzoic acid, Camphos, Dry Ice. Solid CO2 Naphthalene (iii) H2O(g) →H2O(g) (b) (i) Van des waals and hydrogen bonding II Van des waals forces (ii)I. The separation distance is smaller during fusion than during vaporization hence requires much lower energy than in vaporization and vice versa. II. Heating time NP is far much less than heating time in QR/ Heating time (c) (i) Hydrogen burns to produce steam which is a non pollutant/ does not cause pollution to the environment Hydrogen has a high energy content hence very small amount produce a lot of heat energy Hydrogen is renewable hence cannot be exhausted/ used completed. (ii) It can easily explore when burning/ highly flammable unlike fossils fuels expensive. Related Chemistry Questions and Answers on Energy Changes in Chemical and Physical Processes Form 4 Level
0 Comments
Leave a Reply. |
Chemistry Topics
All
Archives
December 2024
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
|
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |

 RSS Feed
RSS Feed

