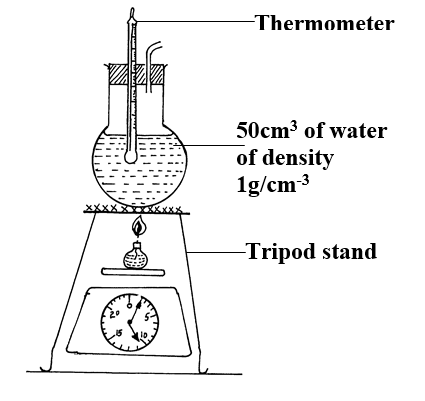
The following set – up was made in an experiment by a group of form four students. The readings of the balance before and after experiment were indicated in the diagram below. Given that the initial temperature of water was 26.7 degrees centigrade respectively. The specific heat capacity of water is 4200Jkg-1k-1
Determine:a) Temperature change that occurred (1mk)
b) Amount of ethanol used (1mk)
10.5 – 1.0 = 9.5g
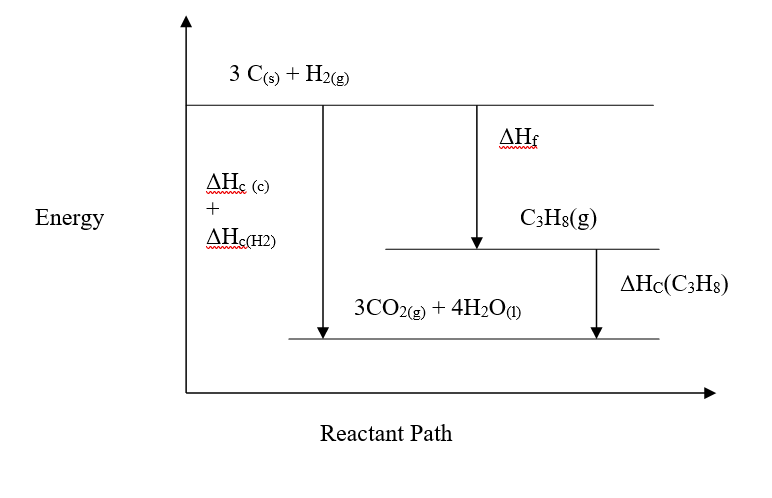
c) Moles of ethanol used (2mks)d) Amount of heat gained by water (2mks)e) Molar enthalpy of combustion of ethanol (2mks)f) Use the following thermochemical processes to answer the questions that follow;i) Draw an energy level diagram representing the formation and combustion processes of propane, carbon and hydrogen (2mks)
ii) Hence or otherwise, determine the heat of formation of propane (2mks)
0 Comments
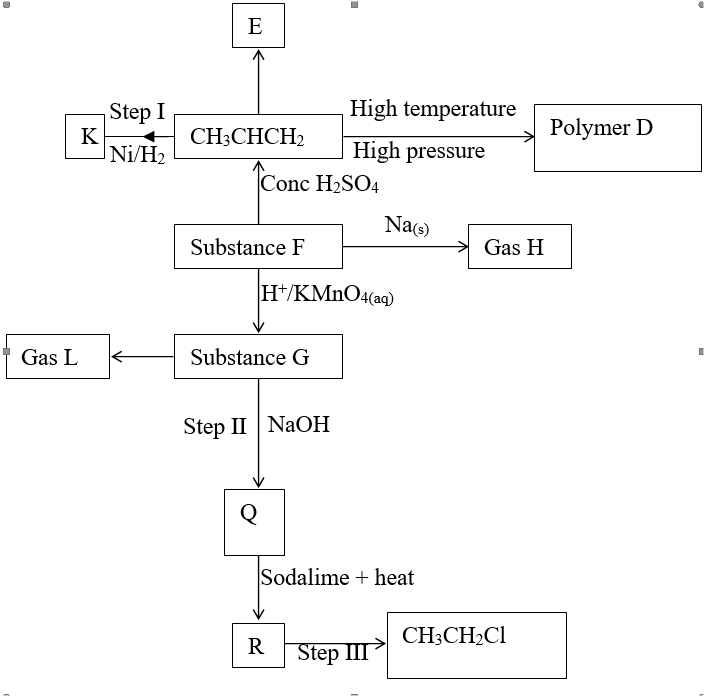
a) Name the following;i) Gas L (1mk)
Gas L – Carbon (IV) Oxide
ii) Gas H (1mk)
Gas H – Hydrogen
iii) K (1mk)
K – Propane
b) Name the processes involved in the following stepsi) Step I (1mk)
Step I : Hydrogen
ii) Step II (1mk)
Step II – Neutralization
iii) Step III (1mk)
Step III – Substitution
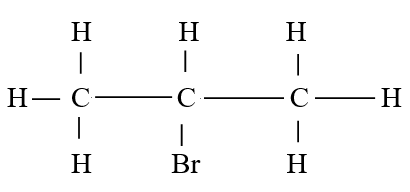
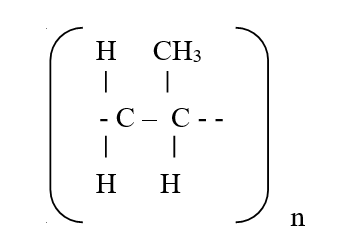
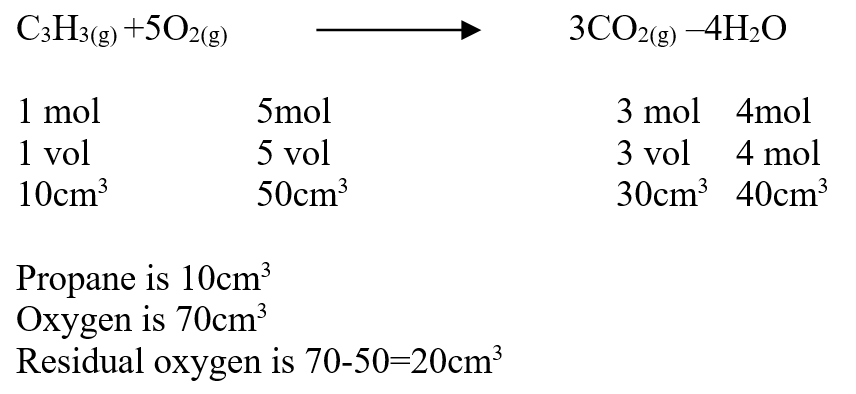
c) Draw the structure of compound E (1mk)d) Write a chemical equation for the complete combustion of substance F (1mk)e) Name the condition and reagents in step IIIi) Condition (1mk)
U.V light
ii) Reagent (1mk)
Chlorine
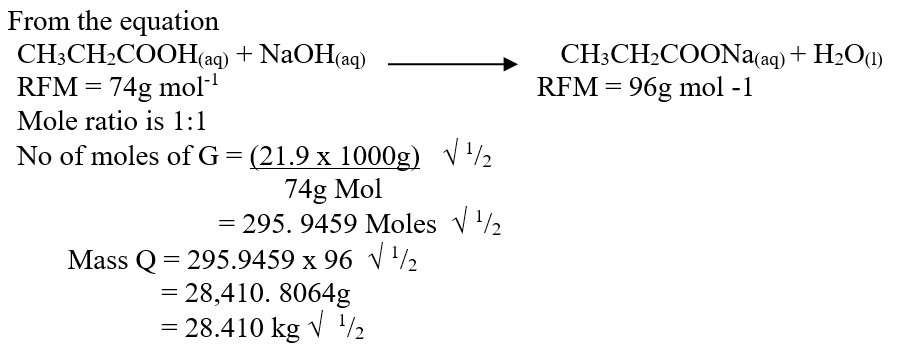
f) Calculate the mass of salt Q that would be formed by using 21.9kg of G when it reacts with excess sodium hydroxide (2mks)
|
Chemistry Topics
All
Archives
December 2024
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
|
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |
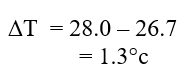
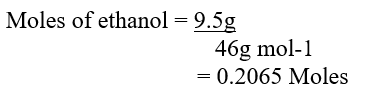
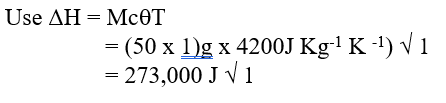

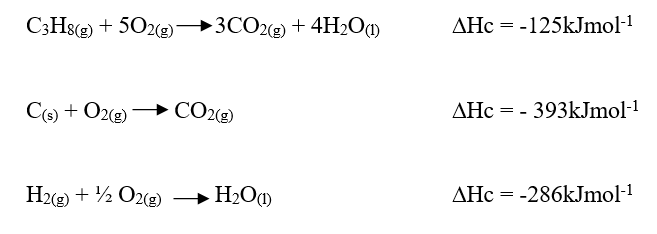
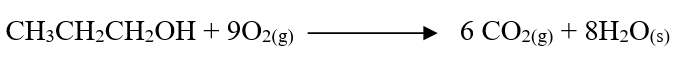

 RSS Feed
RSS Feed

