KCSE CHEMISTRY QUESTIONS AND ANSWERS PER TOPIC
|
These are chemistry questions and answers categorized according to topics, papers i.e. Paper 1 and 2, Levels i.e. form 1 to form 4, kcse year the examination was done and section A or B
Select topic/category to open topical questions from that particular option provided. Chemistry Topics
0 Comments
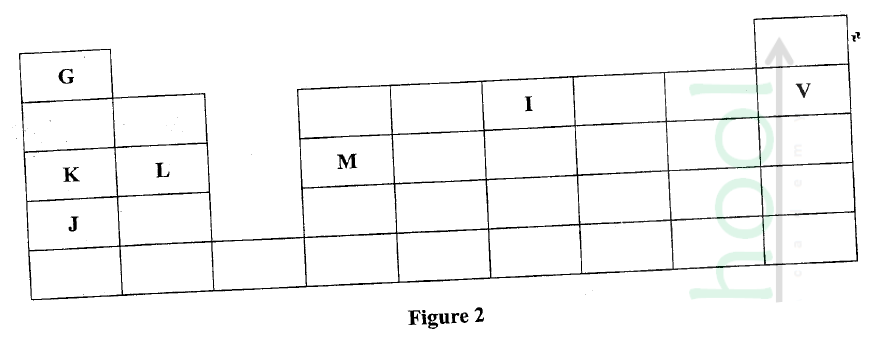
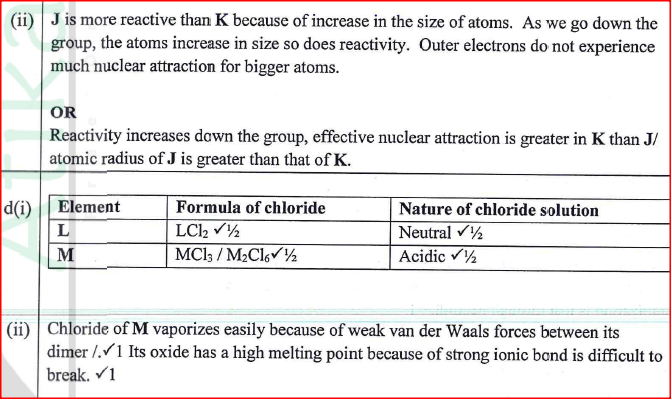
Figure 2 is a section of the periodic table. Study it and answer the questions that follow. The letters do not represent the actual symbols of elements.
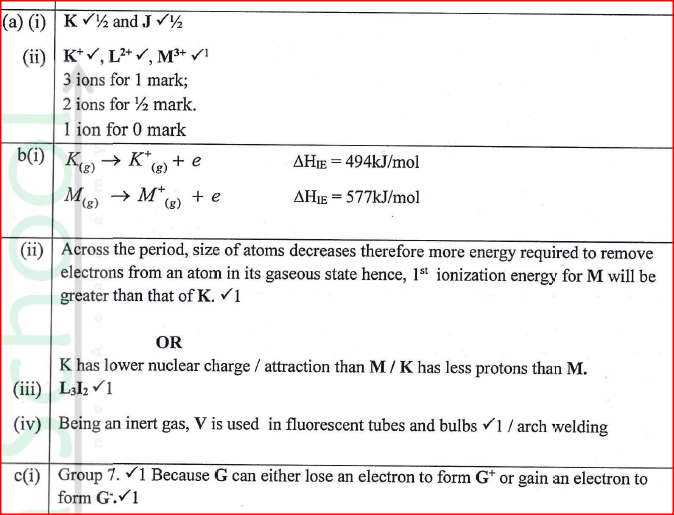
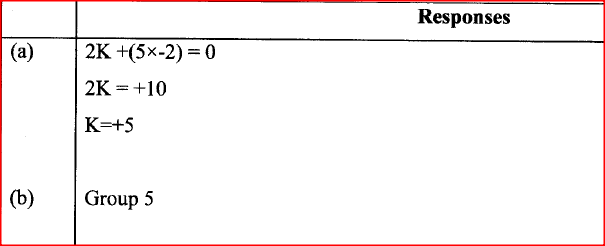
(a) (i) Select elements which belong to the same chemical family.
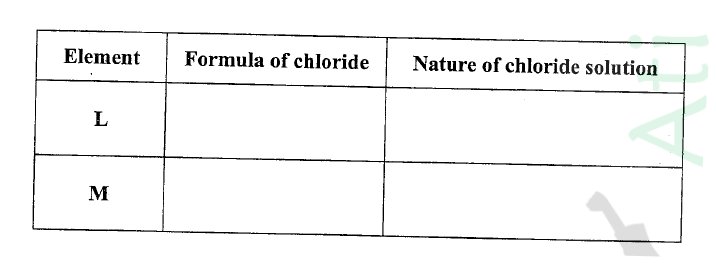
(ii) Write the formulae of ions for elements in the same period. (b) The hrst ionisation energies of two elements K and M at random are 577 kJ/mol and 494 kJ/mol (i) Write equations for the 1ˢᵗ ionisation energies for elements K and M and indicate their energies. (iii) Write the formula of the compound formed when L and I react. (iv) Give one use of elemcnt V. (c) (i) State anothcr group that G can be placcd in Figure 1. Explain. (ii) How do the reactivity of elements J and K compare? Explain. (d) (i) Elements L and M form chlorides. Complete the following table by writing the formulae of each chloride and state the nature of the solutions.
(ii) The chloride of element M vapourises easily while its oxide has a high melting point. Explain.
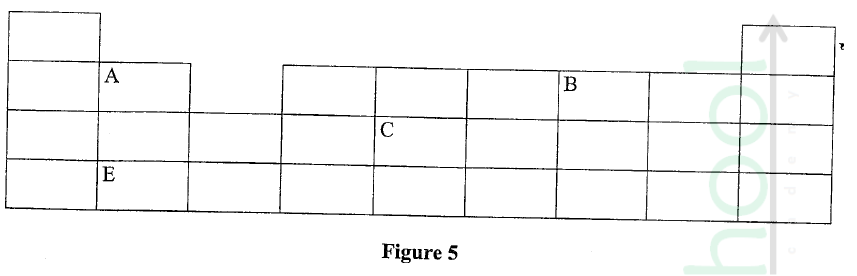
Figure 5 represents a grid that is part of the periodic table. Study it and answer the questions that follow. The letters are not the actual symbols of the elements.
(a) Write the electron arrangement of element C.
(b) On the grid provided, show with a tick (✓) the position of element D whose atomic number is 18. (c) Element E is more reactive than A. Explain.
ANSWERS
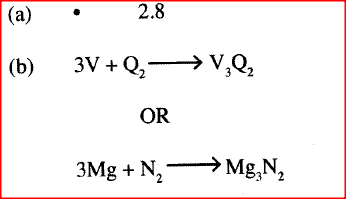
(a) 2.8.4
(b) period 3, group 8 (c) E has a bigger atomic radius than A / the valence electrons of element E are further from the nucleus, hence loosely held by the positive nucleus and requires less energy to be removed during reaction. OR A has a smaller atomic radius than E / the valence electrons of element A are closer to the nucleus, hence strongly held by the positive nucleus and requires more energy to be removed during a reaction.
(a) Element U has atomic number 12 while element V has atomic number 16. How do the melting points of their oxides compare? Explain.
ANSWERS
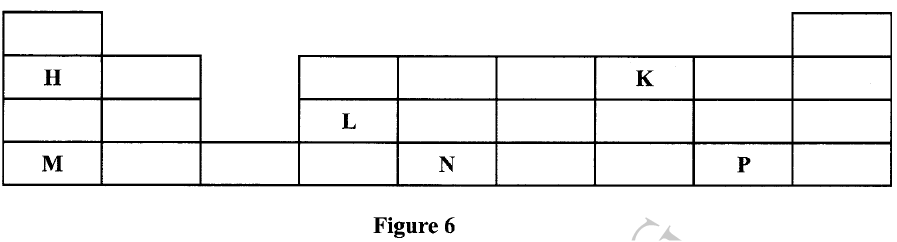
Figure 6 shows part of the periodic table. The letters are not the actual symbols of the elements. Stud it and answer the questions that follow.
(a) Write an equation for the reaction between M and K.
(b) Select the element which can form an ion with a charge of +3. (c) An element J has atomic number 15. Indicate with a tick (✓), on the part of the periodic table the position of J.
An oxide of element K has the formula K2O5.
(a) Determine the oxidation number of K. (b) To which group of the periodic table does K belong?
Table 1 shows the atomic numbers and the first ionisation energies of three elements. The letters are not actual symbols of the elements. Use it to answer the questions that follow.
(a) Explain the trend in first ionisation energy from A to C.
(b) Write the electronic configuration for the ion of C.
ANSWERS
(a) Ionisation energy decreases down the group 1 elements.
This is because atomic radii increases from A to C (down the group) /outermost electron is far from nucleus hence requires less energy to be lost during reaction. (b)Electron configuration of ion of C- 2.8.8
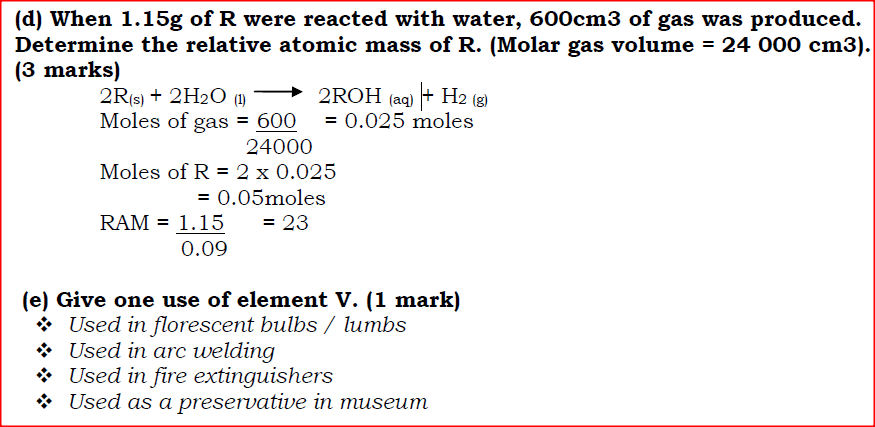
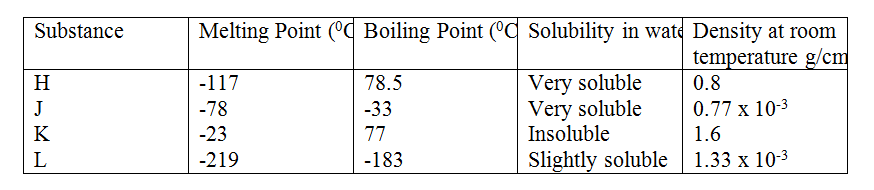
Use the information in the table below to answer the questions that follow. The letters do not represent the actual symbols of the elements.
(a) Give reasons why the melting point of:
(i) S is higher than that of R; (ii) V is lower than that of U. (b) How does the reactivity of W with Chlorine compare with that of R with chlorine? Explain. (c) Write an equation for the reaction between T and excess oxygen. (d) When 1.15g of R were reacted with water, 600cm3 of gas was produced. Determine the relative atomic mass of R. (Molar gas volume = 24 000 cm3). (e) Give one use of element V.
The atomic number of sulphur is 16. Write the electron arrangement of sulphur in the following:
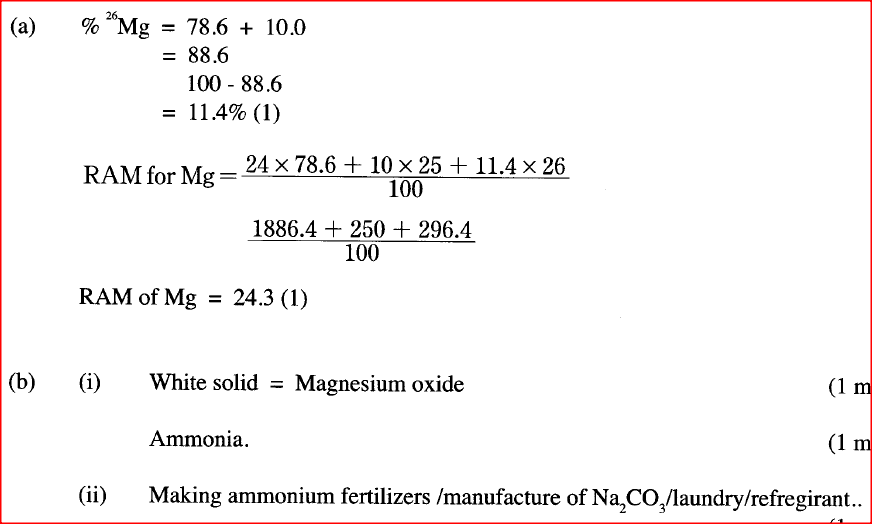
are isotopes of element X. They occur naturally in the ratio of 9:1 respectively.
Calculate the relative atomic mass of element X. (2 marks)
(b) When magnesium burns in air, it forms a white solid and a grey-green solid.
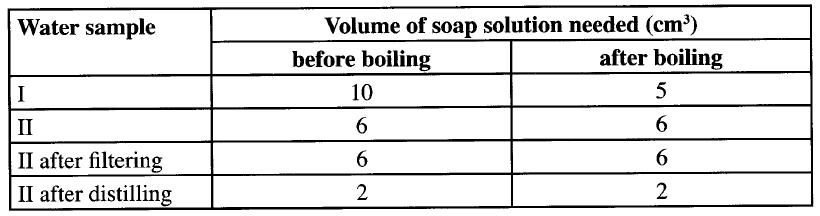
When a few drops of water are added to the mixture, a gas that turns red litmus paper blue is evolved. Identify the (i) white solid. (ii) gas evolved and state its use. (I) Name of gas (II) Use of the gas. ; (c) Two different samples of water (I and II) were tested with soap solution. Sample II was further subjected to two other processes before adding soap. 20 cm3 of each sample of water was shaken with soap solution in a boiling tube until a permanent lather was obtained. The results are shown in the table below
(i) Identify the water sample that had temporary hardness. Explain your answer.
(ii) Explain why the results for sample II are different after distilling but remain unchanged after filtering. (iii) State two disadvantages of using both water samples for domestic purposes.
(a) Name the method that can be used to obtain pure iron (III) chloride from a mixture of iron (III) chloride and sodium chloride.
(b) A student was provided with a mixture of sunflower flour, common salt and a red dye. The characteristics of the three substances in the mixture are given in the table below.
The student was provided with ethanol and any other materials needed.
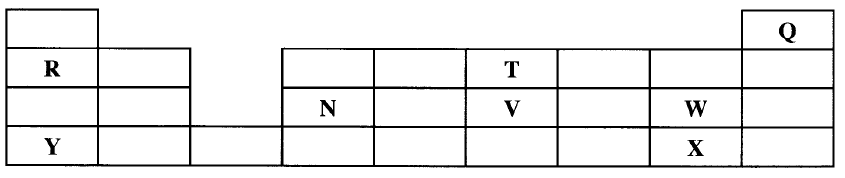
Described how the student can separate the mixture into its three components c) The diagram below show part of a periodic table. The letters do no represent the actual symbols of elements. Use the diagram to answer the questions that follow.
i) Explain why the oxidizing power of W is more than that of X
ii) How do the melting points of R and T compare? Explain iii) Sketch an element that could be used i) In weather ballons ii) For making a cooking pot d i) Classify the substances water, iodine, diamond and candle wax into elements and compounds
ii) Give one use of diamond
ANSWERS
(a)Sublimation
(b)Add ethanol to the mixture . Filter and evaporate filtrate to obtain red dye . Add water to the residue . Filter to obtain sunflower flour . Evaporate filtrate to obtain salt . OR Add H,O to mixture , filter , residue is sunflower , evaporate the water ; add ethanol to the residue filter . The filtrate is red dye. (3 marks) (c)(i) W accepts electrons more readily than X. W has small atomic radius/ W has less energy levels than X/ W has less screening effect than X/ W has greater effective nuclear attraction than X. W is more electro negative than X. (ii) T has a lower melting point than R because it exists in simple molecular form with weak Van der Waals forces while R has strong metallic bonds.
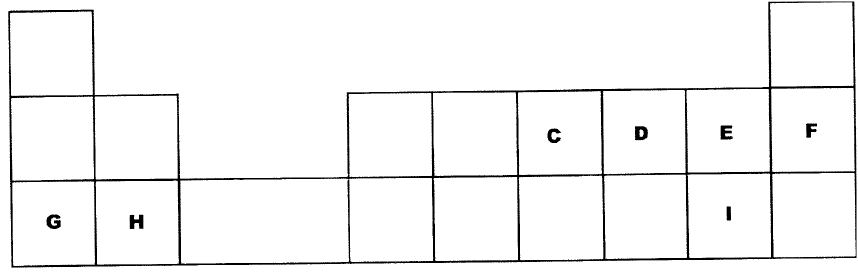
The table below is part of the periodic table. The letters are not the actual symbols of the elements. Study it and answer the questions that follows.
a) Select an element which is stored in paraffin in the laboratory
(b) How do the ionic radii of E and I compare? Explain
ANSWERS
(a) Element stored under paraffin G
(b) E is smaller than I. E has two energy levels while I has 3 energy levels.
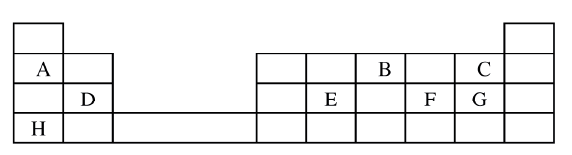
(a) The grid below represents part of the periodic table . Study it and answer the questions that follow. The letters are not the actual symbols of the elements
i) Select the most reactive metal. Explain
ii) Select an element that can form an ion with a charge of 3- iii) Select an alkaline earth metal iv) Which group 1 element has the highest first ionization energy? Explain v) Element A combines with chlorine to form a chloride of A. State the most likely pH value of a solution of a chloride of A. Explain (b) (i) Explain why molten calcium chloride and magnesium chloride conduct electricity while carbon tetrachloride and silicon tetrachloride do not. (ii) Under the same conditions , gaseous neon was found to diffuse faster than gaseous fluorine. Explain this observation. (F=19.0;Ne=20.0)
An atom of element A has mass number 39 and 19 protons.
Explain why hydrogen forms compounds in which its oxidation state is either + 1 or -1 (Atomic number of hydrogen is 1)
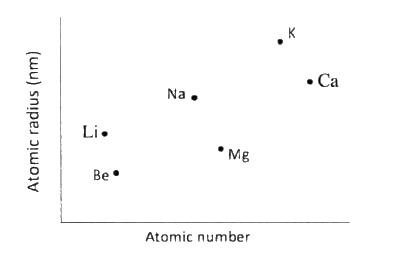
The plots below were obtained when the atomic radii of some elements in groups I and II were plotted against atomic numbers.
Explain:
a) The trend shown by Li, Na and K. b) Why the atomic radii of elements Be, Mg, and Ca are lower than those of Li, Na and K.
ANSWERS
(a)The atomic radii increase with increase in atomic number. This is due to increase in energy levels.
(b) The group II elements have more protons than group I elements hence this increases the nuclear attraction for the outer electrons.
The information in the table below relates to elements in the same group of the periodic table. Study it and answer the question that follows:
Which element has the highest ionization energy? Give reason.
Expected Answer
G3, because it has the smallest atomic radius. Its outer most electron is tightly held by the nucleus or it requires a lot of energy to remove it.
(a) The electronic arrangement of the ion of element Q is 2.8.8. If the formula of the ion is Q3-,state the group and period to which Q belongs.
Group: Period: (b) Helium, neon and argon belong to group 8 of the periodic table. Give: (i) the general name of these elements; (ii) one use of these elements.
(a) The grid given below represents part of the periodic table. Study it and answer the questions that follow. (the letters do not represents the actual symbol of the elements)
(b) Study the information in the table below and answer the questions that follow (the letters do not represent the actual symbol of the substances)
II. By downward displacement of air? (Density of air is 1.29 x 10-3g/cm3 at room temperature. Related Chemistry Questions and Answers on Chemical Families Form 2 Level
Give two reasons why helium is used in weather balloons
Expected Response
The information below relates to elements S,T,U and X. ( the letters do not represents the actual symbols of the elements.
Both T and X are in group II of the periodic Table) (b) Arrange the elements in order of their increasing reactivity
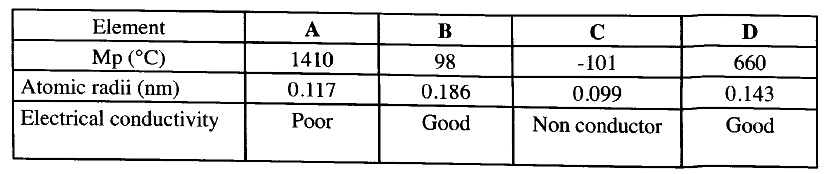
The table below shows properties of some elements A, B, C and D which belong to the same period of the periodic table. The letters are not the actual symbols of the elements.
(a) Arrange the elements in the order they would appear in the period. Give a reason.
(b) Select the metallic element which is the better conductor of electricity. Give a reason.
ANSWERS
(a) BDACAcross the period the atomic radius decreases.
(b) D Across the period the conductivity increase due to increase in delocalised electrons.
The table below gives some properties three elements in group (VII) of the periodic table .Study it and answer the questions that follow:
(a) Which element is in liquid form at room temperature? Give a reason.
(b) Explain why the boiling point of iodine is much higher than that of chlorine.
ANSWERS
(a) Bromine M
At room temperature (25°C), Bromine is liquid since its MP and BP is between -7 and 59 (b) Atomic mass of iodine is higher than that of chlorine. Van der waal’s forces are stronger in iodine than chlorine hence iodine’s BP is higher than that of chlorine.
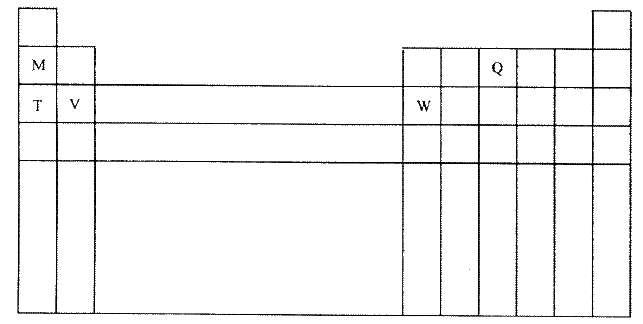
The diagram below represents part of the periodic table. Use it to answer the questions that follow.
a) Write the electronic arrangement for the stable ion formed by W.
(b) Write an equation for the reaction between V and Q. (c) How do the ionization energies of the elements M and T compare? Explain. |
Chemistry Topics
All
Archives
December 2024
|
We Would Love to Have You Visit Soon! |
Hours24 HR Service
|
Telephone0728 450425
|
|
8-4-4 materialsLevels
Subjects
|
cbc materialsE.C.D.E
Lower Primary
Upper Primary
Lower Secondary
Upper Secondary
|
teacher support
Other Blogs
|
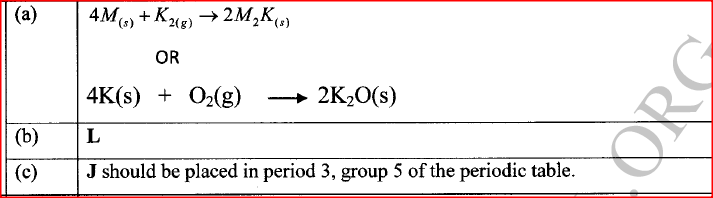





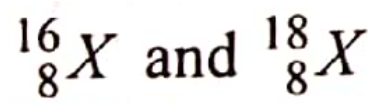













 RSS Feed
RSS Feed