K.C.S.E Biology Q & A - MODEL 1999PP1QN15
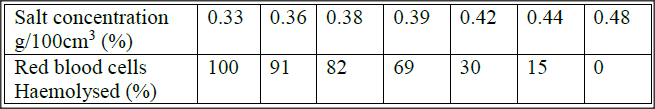
An experiment was carried out to investigate haemolysis of human red blood cells. The red blood cells were placed in different concentrations of sodium chloride solution. The percentage of haemolysed cells was determined. The results were as shown in the table below.
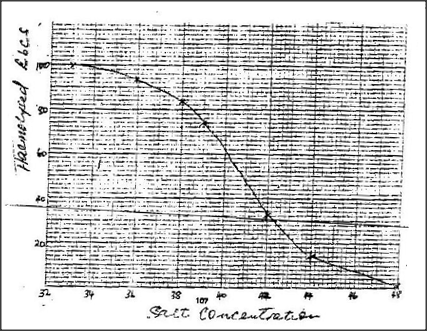
a) i) On the grid provided, plot a graph of harmolysed red blood cells against salt concentration.
ii) at what concentration of salt solution was the proportion of haemolysed cells equal to non-haemolysed cells? iii) State the percentage of cells haemolysed at salt concentration of 0.45% b) Account for the results obtained at: i) 0.33 percent salt concentration. ii) 0.48 percent salt concentration. c) What would happen to the red blood cells if they were placed in 0.50 percent salt solution? d) Explain what would happen to onion epidermal cells if they were placed in distilled water.
answers
a) (i)0.403; 0.404; + 0.002
ii) 0.402; iii) 9-10-11% b) Account for the results obtained at: (i) 0.33 percent salt concentration. Less concentration /hypotonic / dilute than blood cells cytoplasm/ red blood cells; water is drawn in by osmosis the cells swells and eventually burst. (ii)0.48 (ii)0.48 percent salt concentration. Concentration of cytoplasm same as concentration of salt solution/isotonic; therefore no net movement of water; hence no heomolysis. c)Percentage of cells haemolysed would still be zero? Becomes turgid; but does not burst; due to the cell wall. d)The cells would absorb water due to osmosis, swell and become turgid. The cell sap move conc. than surrounding water gate into the cell by osmosis; the cell swells/becomes turgid; but does not burst due to the cell wall
0 Comments
Leave a Reply. |
Archives
December 2024
Categories
All
TOPICSFORM 1
Form 2
Form 3
Form 4
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
|
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |






 RSS Feed
RSS Feed

