|
(a) Write the structural formula of:
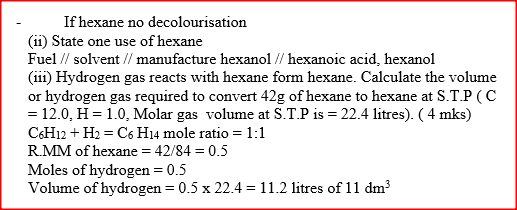
(i) Methanol (ii)Methanoic acid (b) Write the equation for the reaction between methanoic acid and aqueous sodium hydroxide (c) (i) Name the product formed when methanol reacts with methanoic acid (ii) State one condition necessary for the reaction in (c) (i) above to take place (d) (i) Describe one chemical test that can be used to distinguish between hexane and hexane (ii) State one use of hexane (iii) Hydrogen reacts with hexane to form hexane. Calculate the volume or hydrogen gas required to convert 42g of hexane to hexane at S.T.P ( C=12.0, H=1.0, molar gas volume at S.T.P is = 22.4 litres)
0 Comments
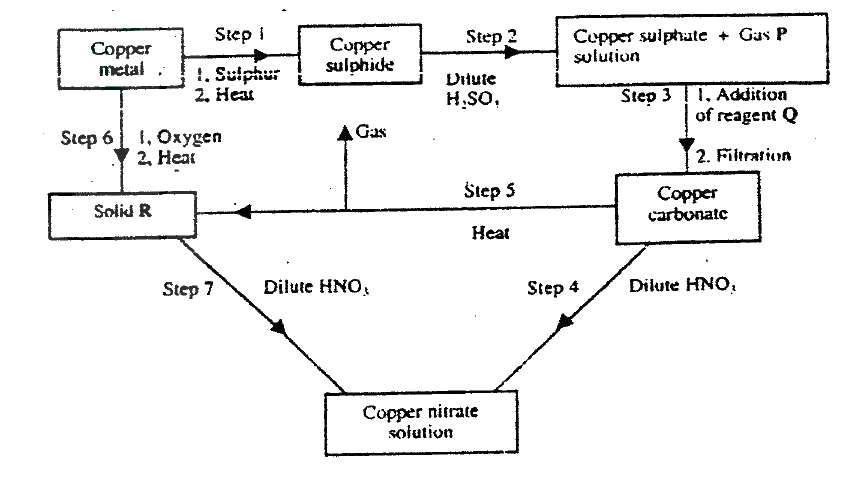
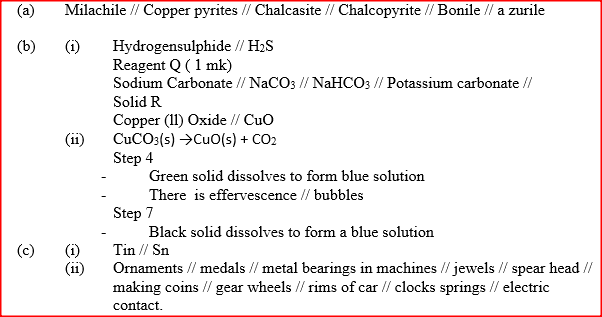
(a) Name one ore from which copper metal is extracted
(b) The chart below shows a sequence of reactions starting with copper. Study it and answer the questions that follow
(ii) Write an equation for the reaction that takes place in step 5
(iii) State the observations made in steps 4 and 7 Step 4 Step 7 (c) Bronze is an alloy of copper and another metal (i) Name the other metal (ii) Give one use of Bronze
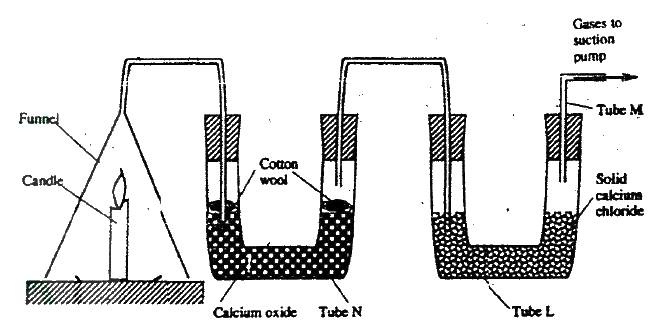
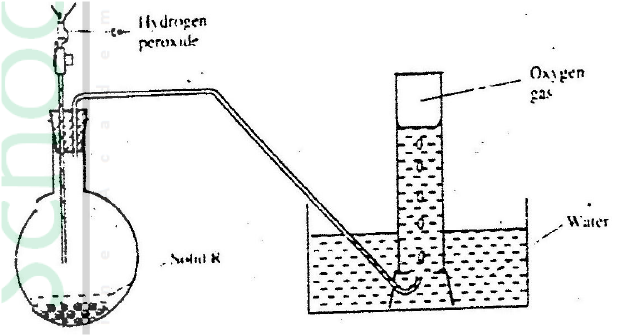
(a) Candle wax is mainly a compound consisting of two elements.
Name the two elements (b) The set- up below was used to investigate the burning of a candle study it and answers the questions that follow
(i) What would happen to the burning candle if the pump was turned off? Give reasons
(ii) State and explain the changes in mass that are likely to occur in tube N by the end of the experiment (iii) Name two gases that come out through tube M (iv) Name another substance that could be used in the place of calcium oxide in tube N
The table below gives standard electrode potentials for the metals represented by the Letters D, E, F and G. study it and answer the questions that follow.
(a) Which metal can be displaced from a solution of its salts by all the other metals in the table? Give a reason
(b) Metals F and G were connected to form a cell as shown in the diagram below
(i) Write the equation for the reactions that occur at electrodes
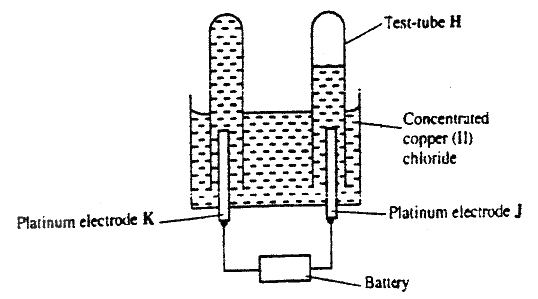
F G (ii) On the diagram, indicate with an arrow the direction in which electrons would flow on the diagram above (ii) What is the function of the salt bridge? (c) An electric current was passed through a concentrated solution of copper (II) chloride as shown in the diagram below
(i) Explain the observation that would be made on the electrolyte as the experiment progresses
(ii) After sometime, test- tube H was found to contain a mixture of two gases. Explain this observation (iii) Which of the electrodes is the anode? Explain
(a) Distinguish between exothermic and endothermic reaction
(b) Changes of state are either exothermic or endothermic Name a change of state that is: (i) Endothermic (ii) Exothermic (c) When pure water is heated at 1 atmospheric pressure at sea level, the temperature of the water does not rise beyond 100°C. Even with continued heated. Explain this observation.
(d) Study the energy cycle diagram below and answer the questions that follow
(i) What does ΔH1 represent? (ii) Show the relationship between ΔH1, ΔH2 and ΔH3 (e) Butane and propane are constituents of a cooking gas. Which produces more energy per mole on combustion? Explain
Study the flow chart below and answer the questions follow
(a) Identify substance
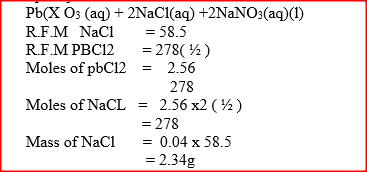
(i) A (ii) B (b) Name process C (c) Give one use of PVC (d) Write an equation for the reaction in which chlorine gas is produced (e) State and explain the observation that would be made if chlorine gas was bubbled into an aqueous solution of sodium iodide (f) In the preparation of a bleaching agent (Sodium hypochlorite), Excess chlorine gas was bubbled into 15 litres of cold 2 m sodium hydroxide (i) Write an equation for the reaction between chlorine gas and cold dilute sodium Hydroxide (ii) Calculate the: Number of moles of sodium hydroxide used Mass in kilograms of the sodium hypochlorite produced = 1. 1175
(a) what method can be used to separate a mixture of ethanol and propanol?
(b) (i) Explain how a solid mixture of sulphur and sodium chloride can be separated Into solid sulphur and solid chloride (c) The table below gives the solubilities of potassium bromide and potassium sulphate at 00C and 400C
When an aqueous mixture containing 60g of potassium and 7 g of potassium sulphate in 10g of water at 800C was cooled to 00C some crystals were formed
(i) Identify the crystals (ii) Determine the mass of the crystals formed (iii) Name the method used to obtain the crystals (iv) Suggest one industrial application of the method named in (iii) above
The following tests were carried out on three separate portions of a colourless solution S
(a) From the information in test (i), name a cation, which is not present in solution S.
(b) Identify a cation, which is likely to be present in solution S (c) Write an ionic equation for the reaction, which takes place in test (ii)
But -2- ene undergoes hydrogenation according to the equation given below
CH3CH = CHCH3 (g) + H2 (g) →CH3CH2CH2CH3 (g) (a) Name the product formed when but -2 – ene reacts with hydrogen gas (b) State one industrial use of hydrogenation
answers
a)Butane
b)Hardening of oils in the manufacture of margarine
(a) Give a reason why concentrated sulphuric acid is not used to dry ammonia gas
(b) Name one suitable drying agent for ammonia gas
answers
a)Being acidic, it would react with the basic ammonia
b)CaO
In an experiment 30cm3 of 0.1 M sulphuric acid were reacted with 30cm3 of 0.1 M sodium hydroxide
(a) Write in equation of the reaction that took place (b) State the observations that were made when both and red litmus papers were dropped into the mixture (c) Give a reason for your answer in (a) above
answer
(a) Solid dissolves
(b) addition of hydrochloric acid favour backward reaction
Describe how a solid sample of Zin (II) carbonate can be prepared starting with zinc oxide
The structures below represents a portion of a polymer
(a) Give the name of the polymer (b) Give one industrial use of the polymer
answer
With reference to iodine, distinguish between covalent bonds and Van Der Waals forces
answers
When carbon dioxide gas was passed through aqueous calcium hydroxide a white suspension was formed
(a) Write an equation for the reaction that took place (b) State and explain the changes that would occur when carbon dioxide gas is bubbled through the white suspension
Iron is extracted from its ore by the blast furnace process
(a) Name one ore from which iron is extracted (b) One of the impurities in iron is removed in the form of calcium silicate. Write an equation for the reaction in which calcium silicate is produced
Concentrated nitric (V) acid was added to iron (II) sulphate acidified with sulphuric acid and the mixture heated. The solution turned from pale green to yellow with evolution of brown gas. Explain these observations.
answer
In an experiment, sulphur dioxide gas was bubbled into water followed by chlorine gas. The resulting clear solution gave a white precipitate when mixed with a acidified barium chloride solution. Explain these observations
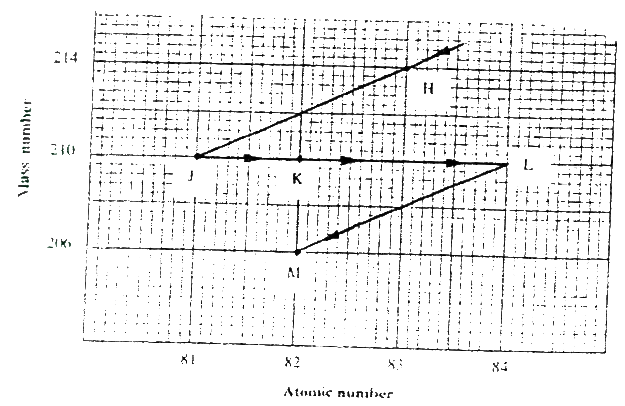
The graph below represents a radioactive decay series for isotope H. Study it and answer the questions that follow
(a) Name the type of radiation emitted when isotope H changes to isotope J.
(b) Write an equation for the nuclear reaction that occur when isotope J changes to isotope K
(c) Identify a pair of isotope of an element in the decay series
|
Chemistry Topics
All
Archives
December 2024
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
|
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |












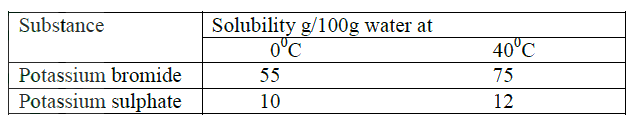


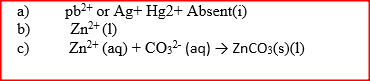


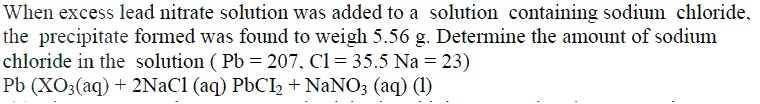


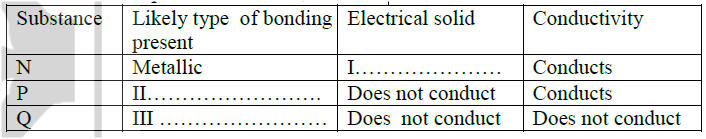


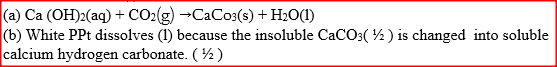
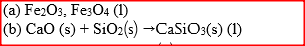



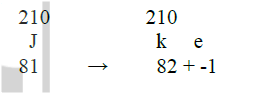
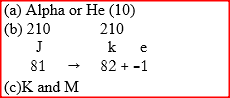

 RSS Feed
RSS Feed

