Comprehensive KCSE Form 2 Chemistry Notes for Effective LearningReview:
These KCSE Form 2 Chemistry Notes are a valuable resource for students studying chemistry at the secondary level. The document covers essential topics such as atomic structure, periodic table trends, isotopes, and relative atomic mass. The content is presented in a clear and concise manner, making it easy for students to understand and apply the concepts. The notes provide detailed explanations of the three subatomic particles (protons, electrons, and neutrons) and their relative positions within an atom. The periodic table is also thoroughly discussed, emphasizing the trends across and down the table. This knowledge is essential for understanding chemical bonding and structure. One of the strengths of these notes is the inclusion of examples and calculations. This allows students to apply the concepts they have learned and practice their problem-solving skills. The document also provides a table showing the atomic structure of the first twenty elements, which serves as a useful reference. Furthermore, the notes explain the concept of isotopes and how to calculate the relative atomic mass using the relative abundance of isotopes. The examples provided help students grasp this concept effectively. Additionally, the document highlights the importance of the mass spectrometer in determining accurate relative atomic masses. Overall, these KCSE Form 2 Chemistry Notes are a comprehensive and well-structured resource for students. They cover all the necessary topics and provide ample examples for practice. By using these notes, students can build a strong foundation in chemistry and excel in their studies.
1 Comment
Chemistry Practical Guide: Qualitative and Quantitative Analysis TechniquesReview
The Chemistry Practical Guide is a comprehensive resource that provides students with specific objectives and techniques for conducting chemistry experiments. The guide aims to test students' abilities to select and handle apparatus, make accurate observations, and draw conclusions based on those observations. It focuses on three main areas: qualitative analysis, quantitative analysis, and graphical work. Qualitative analysis involves performing chemical tests to identify substances. To obtain accurate results, students need to accurately identify the test reagents, understand what these reagents test, and predict the expected results. One of the key aspects of qualitative analysis is testing for cations, which involves adding certain reagents to a solution and noting the resulting observations and inferences. The guide provides a detailed list of cations and their corresponding observations and inferences. Another important aspect of qualitative analysis is testing for anions. This involves adding specific reagents and observing the resulting precipitates or color changes. The guide outlines the steps and observations for testing six common anions. Quantitative analysis focuses on making accurate measurements. It involves techniques such as titration and gravimetric analysis to determine the concentration or amount of a substance in a given sample. The guide provides an overview of these techniques and their applications. Graphical work involves the interpretation and analysis of data using graphs and charts. It includes techniques such as plotting graphs, determining gradients, and analyzing trends. Overall, the Chemistry Practical Guide serves as a valuable resource for students to enhance their understanding and skills in experimental chemistry. It provides clear instructions, observations, and inferences for qualitative and quantitative analysis techniques. By following the guide, students can develop the necessary proficiency in handling apparatus, making accurate observations, and drawing conclusions. With a strong foundation in practical chemistry, students will be well-prepared for their examinations and future scientific endeavors. Comprehensive Chemistry Form 1-4 Notes for Effective LearningReview:
Our Chemistry Form 1-4 Notes are an invaluable resource for students seeking a thorough understanding of the subject. The notes cover a wide range of topics, from the states of matter to the role of chemistry in society. Each concept is explained in a clear and concise manner, making it easy for students to grasp even the most complex ideas. One of the strengths of these notes is the inclusion of practical examples, which help students apply their knowledge to real-world scenarios. For example, the section on separation of mixtures provides simple methods that can be used to separate different substances, allowing students to see the practical applications of their learning. Furthermore, the notes emphasize the importance of safety in the chemistry laboratory. Clear guidelines are provided to ensure that students understand how to handle chemicals safely and minimize the risk of accidents. This focus on safety is crucial in fostering responsible and conscientious scientific practices. The layout and organization of the notes are also commendable. Each topic is presented in a logical sequence, building upon previous knowledge and paving the way for a smooth progression of learning. The use of headings, subheadings, and bullet points makes it easy for students to navigate through the content and locate specific information. Overall, our Chemistry Form 1-4 Notes provide a comprehensive and well-structured resource for students studying chemistry. With its clear explanations, practical examples, and emphasis on safety, this resource is sure to enhance students' understanding and appreciation of the subject. Comprehensive Chemistry Summary Notes for Form 1 to Form 4Review: The "FORM 1 TO FORM 4 CHEMISTRY SUMMARY NOTES.pdf" is an invaluable resource for students studying chemistry at the Form 1 to Form 4 level. The notes provide a comprehensive overview of the entire chemistry syllabus, covering all the key topics and concepts. The document is well-organized, with each topic clearly outlined and explained in a concise manner. The content is presented in a way that is easy to understand, making it suitable for students of all levels of ability. The notes include detailed explanations, diagrams, and examples to help students grasp the fundamental principles of chemistry. One of the standout features of these notes is the inclusion of practical applications and real-life examples. This helps students to see the relevance of chemistry in everyday life and enhances their understanding of the subject. The document also includes a section on exam tips and strategies, which is particularly helpful for students preparing for their chemistry exams. It provides guidance on how to approach different types of questions, how to structure answers, and how to effectively revise for exams. Overall, the "FORM 1 TO FORM 4 CHEMISTRY SUMMARY NOTES.pdf" is a comprehensive and well-structured resource that covers all the essential topics in chemistry. Whether you are a student looking for additional study material or a teacher seeking a reference guide, these notes are a valuable asset. Using dots (•) and cross (x) show the formation of Carbon (II) oxide gas (1mk)Name two types of bonds present in the molecule in ‘a’ above (2mks)
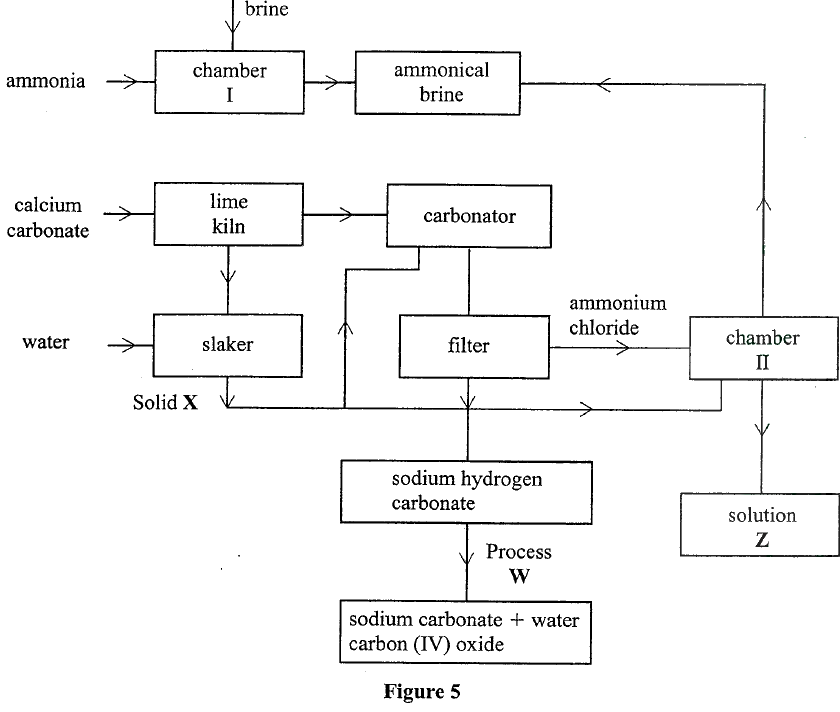
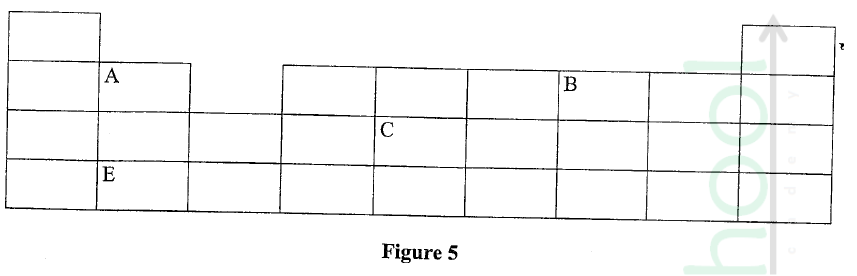
(a) In Kenya, sodium carbonate is extracted from trona at Lake Magacli.
(i) Give the formula of trona. (ii) Name the process of extracting sodium carbonate from trona. (b) The flow chart in Figure 5 summarises the steps involved in the production of sodium carbonate. Use it to answer the questions that follow.
(i) Name the process illustrated in Figure 5.
(ii) Identify the starting raw materials required in the production of sodium carbonate. (iii) Write equations for the two reactions that occur in the carbonator. (iv) Name two substances that are recycled. (v) Identify: Solid X; Process W. (vi) Write an equation for the reaction that produces solution Z. (vii) Apart from softening hard water, state two other uses of sodium carbonate.
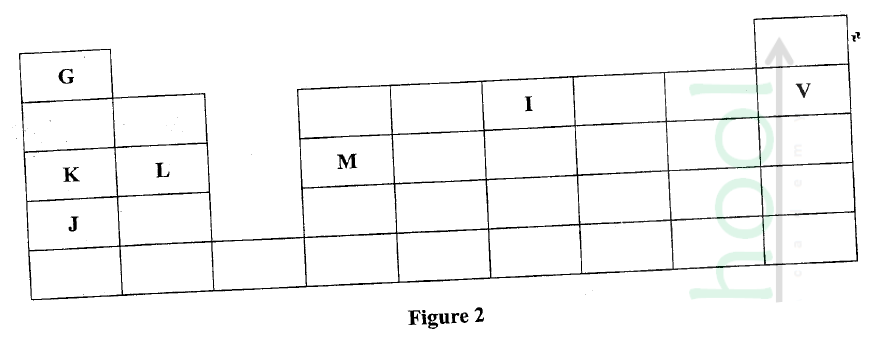
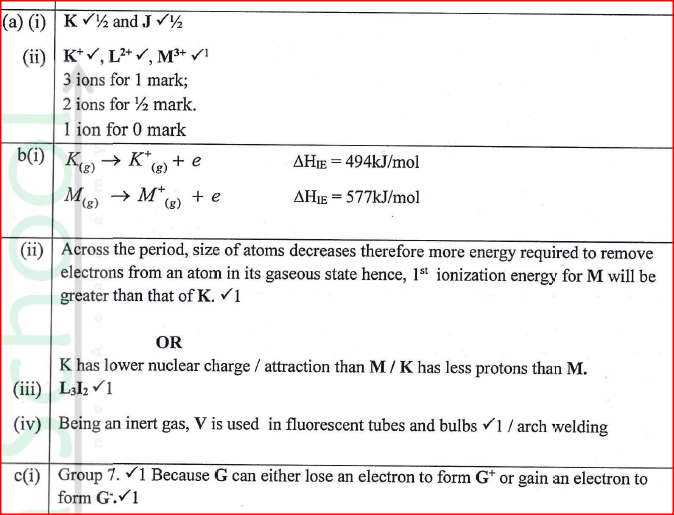
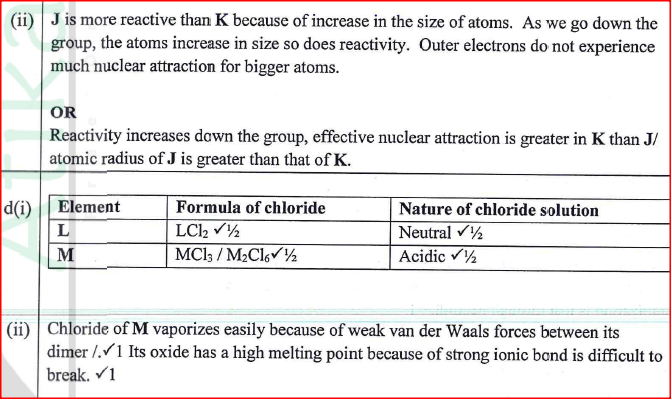
Figure 2 is a section of the periodic table. Study it and answer the questions that follow. The letters do not represent the actual symbols of elements.
(a) (i) Select elements which belong to the same chemical family.
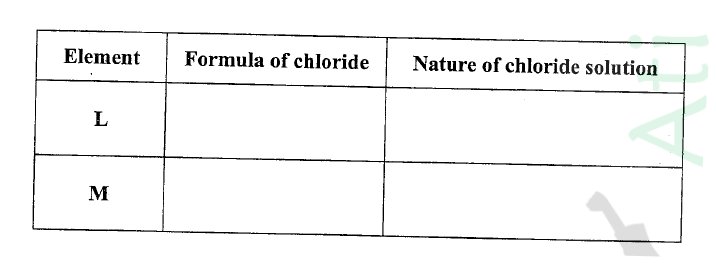
(ii) Write the formulae of ions for elements in the same period. (b) The hrst ionisation energies of two elements K and M at random are 577 kJ/mol and 494 kJ/mol (i) Write equations for the 1ˢᵗ ionisation energies for elements K and M and indicate their energies. (iii) Write the formula of the compound formed when L and I react. (iv) Give one use of elemcnt V. (c) (i) State anothcr group that G can be placcd in Figure 1. Explain. (ii) How do the reactivity of elements J and K compare? Explain. (d) (i) Elements L and M form chlorides. Complete the following table by writing the formulae of each chloride and state the nature of the solutions.
(ii) The chloride of element M vapourises easily while its oxide has a high melting point. Explain.
Explain why a solution of sodium chloride Conducts electricity while that of sugar does not.
ANSWERS
Figure 5 represents a grid that is part of the periodic table. Study it and answer the questions that follow. The letters are not the actual symbols of the elements.
(a) Write the electron arrangement of element C.
(b) On the grid provided, show with a tick (✓) the position of element D whose atomic number is 18. (c) Element E is more reactive than A. Explain.
ANSWERS
(a) 2.8.4
(b) period 3, group 8 (c) E has a bigger atomic radius than A / the valence electrons of element E are further from the nucleus, hence loosely held by the positive nucleus and requires less energy to be removed during reaction. OR A has a smaller atomic radius than E / the valence electrons of element A are closer to the nucleus, hence strongly held by the positive nucleus and requires more energy to be removed during a reaction.
You are provided with solid potassium hydrogen carbonate. Describe how a solid sample of potassium nitrate can be prepared.
ANSWERS
Measure a certain volume of dilute nitric(V) acid and place it in a beaker;
Add potassium hydrogen carbonate little by little as the mixture is stirred until effervescence stops; Evaporate the solution to saturation and allow to cool for crystals to form; Dry the crystals in between filter papers.
(a) Element U has atomic number 12 while element V has atomic number 16. How do the melting points of their oxides compare? Explain.
ANSWERS
(a) Explain why it is not advisable to prepare a sample of carbon(IV) oxide using barium carbonate and dilute sulphuric(VI) acid.
(b) State a method that can be used to collect dry carbon(IV) oxide gas. Give a reason.
ANSWERS
(a)The reaction starts but soon stops.
This is because the insoluble barium sulphate produced forms a coating on the surface of the barium carbonate preventing further reaction and evolution of carbon(IV) oxide gas. (b) Downward delivery. Carbon(TV) oxide is denser than air.
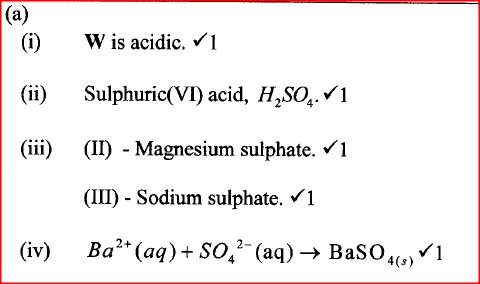
W is a colourless aqueous solution with the following properties:
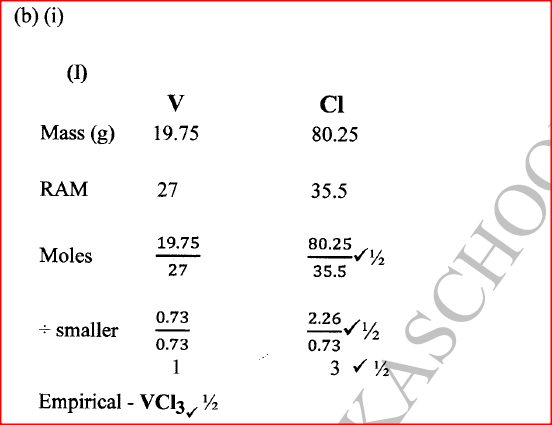
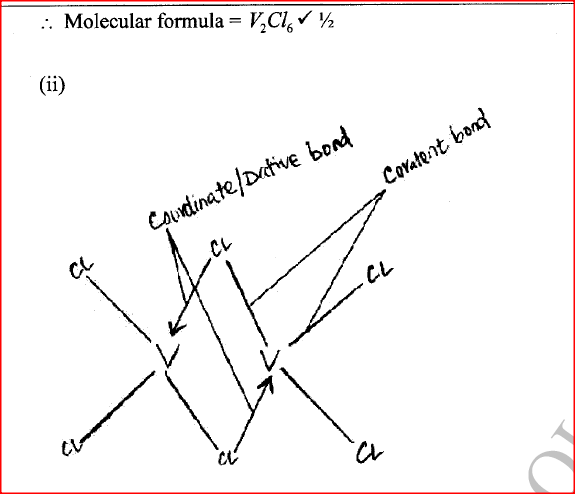
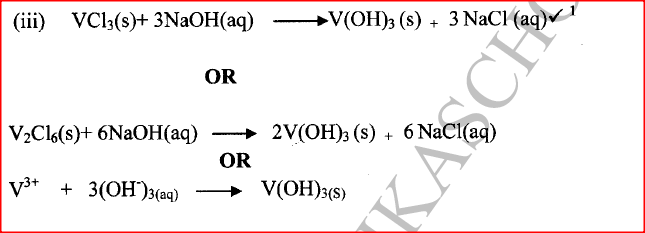
(ii) Give the identity of W. (iii) Name the colourless solution formed in (II) and (III). (iv) Write an ionic equation for the reaction indicated in (V). (b) Element V conducts electricity and melts at 933K. When chlorine gas is passed over heated V, it forms a vapour that solidifies on cooling. The solid chloride dissolves in water to form an acidic solution. The chloride vapour has a relative molecular mass of 267 and contains 19.75% of V. At a higher temperature, it dissociates to a compound of relative molecular mass 133.5. When aqueous sodium hydroxide is added to the aqueous solution of the chloride, a white precipitate is formed which dissolves in excess alkali. (V = 27.0 ; CI = 35.5) (i) Determine the:
(iii) Write an equation for the reaction that form a white precipitate with sodium hydroxide.
In terms of structure and bonding, explain why graphite is used as a lubricant in machines.
ANSWERS
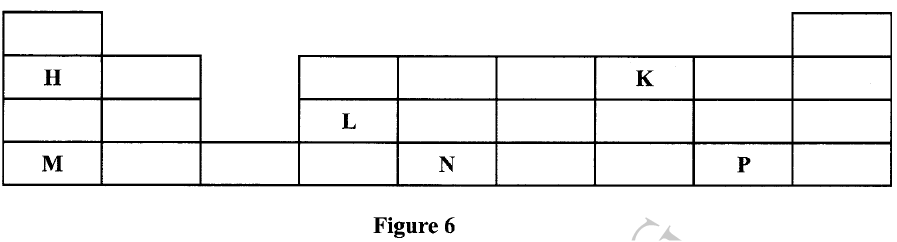
Figure 6 shows part of the periodic table. The letters are not the actual symbols of the elements. Stud it and answer the questions that follow.
(a) Write an equation for the reaction between M and K.
(b) Select the element which can form an ion with a charge of +3. (c) An element J has atomic number 15. Indicate with a tick (✓), on the part of the periodic table the position of J.
The atomic numbers of some elements P, Q, R and S are 6, 8, 12 and 17 respectively.
(a) Draw the dot (•) and cross (X) diagrams for the compounds formed when: (i) R and Q react (ii) P and S react. (b) Explain why the melting point of the compound formed by P and S is lower than that formed by R and Q.
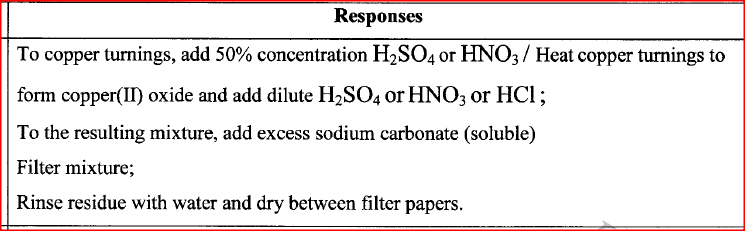
Starting with copper, describe how a pure sample of copper(II) carbonate can be prepared.
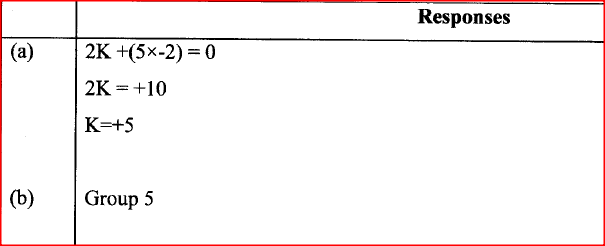
An oxide of element K has the formula K2O5.
(a) Determine the oxidation number of K. (b) To which group of the periodic table does K belong?
Table 1 shows the atomic numbers and the first ionisation energies of three elements. The letters are not actual symbols of the elements. Use it to answer the questions that follow.
(a) Explain the trend in first ionisation energy from A to C.
(b) Write the electronic configuration for the ion of C.
ANSWERS
(a) Ionisation energy decreases down the group 1 elements.
This is because atomic radii increases from A to C (down the group) /outermost electron is far from nucleus hence requires less energy to be lost during reaction. (b)Electron configuration of ion of C- 2.8.8
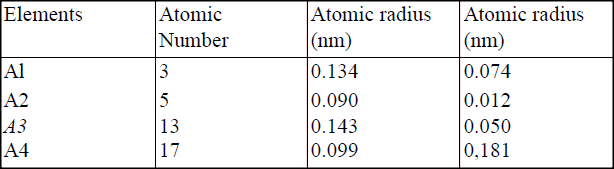
(a) Write an equation to show the effect of heat on the nitrate of:
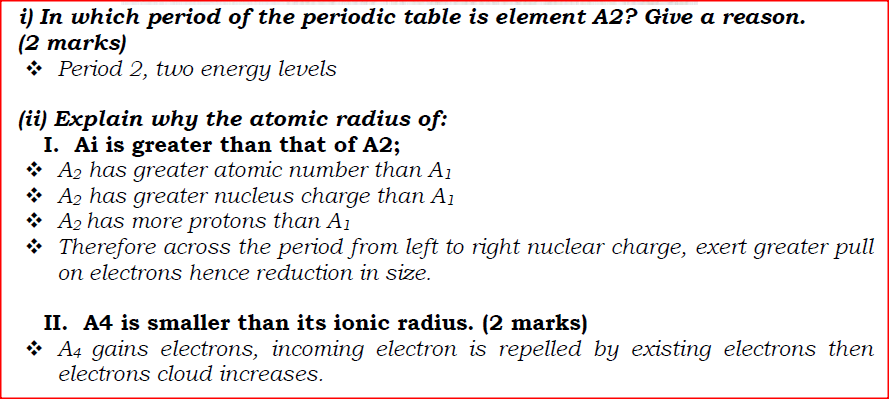
(i) Potassium (ii) Silver (b) The table below gives information about elements Ai, A2, A3, and A4 i) In which period of the periodic table is element A2? Give a reason.
(ii) Explain why the atomic radius of:
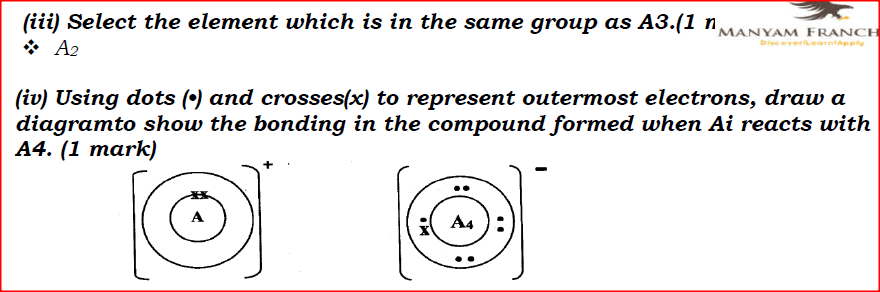
I. Ai is greater than that of A2; II. A4 is smaller than its ionic radius. (iii) Select the element which is in the same group as A3. (iv) Using dots (•) and crosses(x) to represent outermost electrons, draw a diagramto show the bonding in the compound formed when Ai reacts with A4.
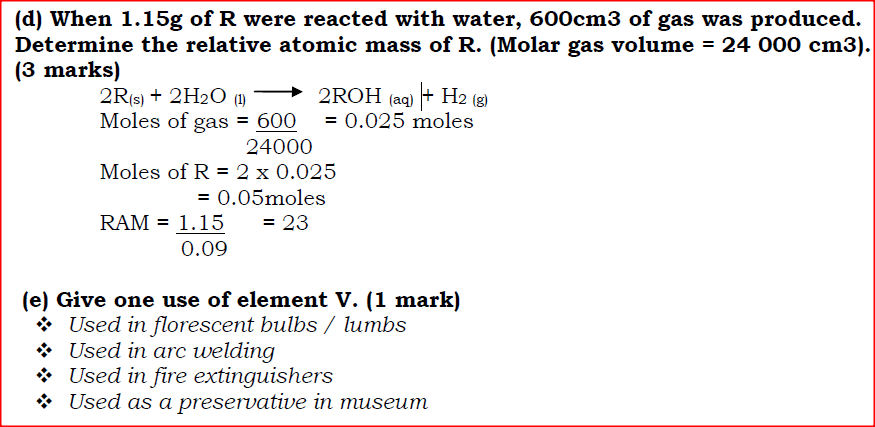
Use the information in the table below to answer the questions that follow. The letters do not represent the actual symbols of the elements.
(a) Give reasons why the melting point of:
(i) S is higher than that of R; (ii) V is lower than that of U. (b) How does the reactivity of W with Chlorine compare with that of R with chlorine? Explain. (c) Write an equation for the reaction between T and excess oxygen. (d) When 1.15g of R were reacted with water, 600cm3 of gas was produced. Determine the relative atomic mass of R. (Molar gas volume = 24 000 cm3). (e) Give one use of element V.
A mixture contains ammonium chloride, copper (II) oxide and sodium chloride. Describe how each of the substances can be obtained form the mixture.
ANSWERS
Charcoal is a fuel that is commonly used for cooking. When it burns it forms two oxides.
(a) Name the two oxides. (b) State one use of the two oxides.
ANSWERS
(a)Carbon (IV) oxide /CO2/ carbon dioxide
Carbon (II) oxide/ CO/ carbon monoxide (b)Fire extinguisher/ photosynthesis Refrigeration Solvay process Fizzy drinks Food preservation Extraction of metals Manufacture of methanol Manufacture of fuel (water, gas)
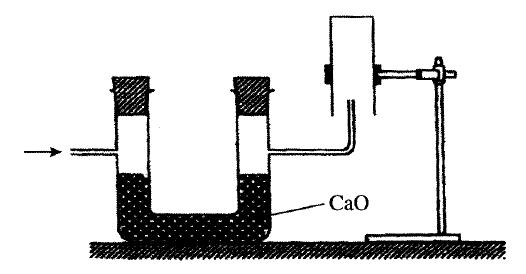
The set-up below was used to collect a dry sample of a gas
Give two reasons why the set-up cannot be used to collect carbon (IV) oxide gas.
ANSWERS
The atomic number of sulphur is 16. Write the electron arrangement of sulphur in the following:
|
Chemistry Topics
All
Archives
December 2024
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
|
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |



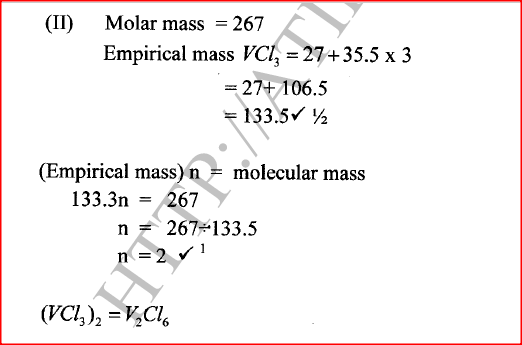
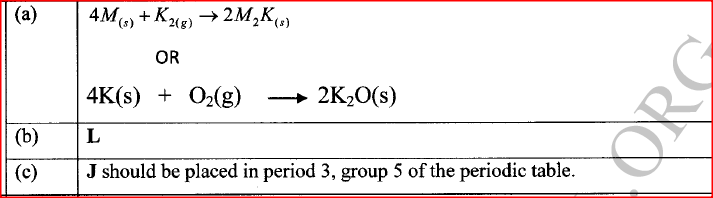



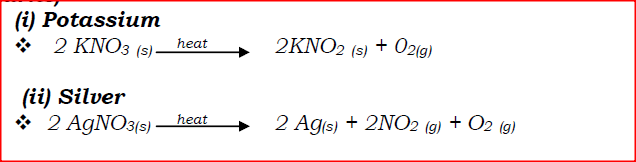





 RSS Feed
RSS Feed

