Introduction to Chemistry: States of Matter, Mixtures, Conductors, and DrugsThis detailed article on the introduction to chemistry covers a wide range of topics, making it a valuable resource for students and enthusiasts alike. The content is well-structured, starting with an overview of the states of matter and their properties. The explanations are clear and concise, making it easy to understand the differences between solids, liquids, and gases. The section on the separation of mixtures provides simple methods that can be easily understood by beginners. The discussion on conductors and non-conductors of electricity is informative, with a list of examples for better comprehension. The article also highlights the role of chemistry in society, emphasizing its importance in various fields such as medicine, manufacturing, and diagnostics. The section on drugs provides a clear distinction between drugs and medicines, highlighting the significance of correct prescription and dosage. Overall, this article is a comprehensive guide to the basics of chemistry and its practical applications, making it a valuable resource for anyone interested in the subject.
0 Comments
Chemistry Practical Guide: Qualitative and Quantitative Analysis TechniquesReview
The Chemistry Practical Guide is a comprehensive resource that provides students with specific objectives and techniques for conducting chemistry experiments. The guide aims to test students' abilities to select and handle apparatus, make accurate observations, and draw conclusions based on those observations. It focuses on three main areas: qualitative analysis, quantitative analysis, and graphical work. Qualitative analysis involves performing chemical tests to identify substances. To obtain accurate results, students need to accurately identify the test reagents, understand what these reagents test, and predict the expected results. One of the key aspects of qualitative analysis is testing for cations, which involves adding certain reagents to a solution and noting the resulting observations and inferences. The guide provides a detailed list of cations and their corresponding observations and inferences. Another important aspect of qualitative analysis is testing for anions. This involves adding specific reagents and observing the resulting precipitates or color changes. The guide outlines the steps and observations for testing six common anions. Quantitative analysis focuses on making accurate measurements. It involves techniques such as titration and gravimetric analysis to determine the concentration or amount of a substance in a given sample. The guide provides an overview of these techniques and their applications. Graphical work involves the interpretation and analysis of data using graphs and charts. It includes techniques such as plotting graphs, determining gradients, and analyzing trends. Overall, the Chemistry Practical Guide serves as a valuable resource for students to enhance their understanding and skills in experimental chemistry. It provides clear instructions, observations, and inferences for qualitative and quantitative analysis techniques. By following the guide, students can develop the necessary proficiency in handling apparatus, making accurate observations, and drawing conclusions. With a strong foundation in practical chemistry, students will be well-prepared for their examinations and future scientific endeavors. Exploring Flames, Apparatus, and Chemical Reactions in ChemistryReview
"Exploring Flames, Apparatus, and Chemical Reactions in Chemistry" is an insightful document that provides a comprehensive understanding of different aspects of chemistry. The document begins by explaining the differences between flames, specifically the non-luminous flames F and G, which were observed during an experiment. It also describes the order and arrangement of apparatus required to investigate the pH of an onion solution. The document further delves into the concept of drugs, defining them and highlighting their abuse by the youth. It identifies three common methods for collecting gases in the laboratory, which are often employed for various experiments. Moreover, the document discusses the separation of mixtures using the example of a mixture of hexane and water. It explains the states and functions of various apparatus commonly used in the laboratory, providing a deeper understanding of the equipment's roles in chemical experiments. The document also sheds light on the appearance of paper pieces in different parts of a non-luminous flame, revealing which region of the flame is better suited for heating. Additionally, the document includes an experiment involving copper (II) sulfate and water, which investigates the ionic radii of different substances. It explains the observations made during the experiment and provides a simple classification of substances based on heating curves. Overall, "Exploring Flames, Apparatus, and Chemical Reactions in Chemistry" is a comprehensive document that covers a wide range of topics in chemistry. It provides clear explanations, diagrams, and examples to enhance understanding. This document is a valuable resource for students, educators, and anyone interested in gaining a deeper knowledge of chemistry and its applications. Comprehensive Chemistry Form 1-4 Notes for Effective LearningReview:
Our Chemistry Form 1-4 Notes are an invaluable resource for students seeking a thorough understanding of the subject. The notes cover a wide range of topics, from the states of matter to the role of chemistry in society. Each concept is explained in a clear and concise manner, making it easy for students to grasp even the most complex ideas. One of the strengths of these notes is the inclusion of practical examples, which help students apply their knowledge to real-world scenarios. For example, the section on separation of mixtures provides simple methods that can be used to separate different substances, allowing students to see the practical applications of their learning. Furthermore, the notes emphasize the importance of safety in the chemistry laboratory. Clear guidelines are provided to ensure that students understand how to handle chemicals safely and minimize the risk of accidents. This focus on safety is crucial in fostering responsible and conscientious scientific practices. The layout and organization of the notes are also commendable. Each topic is presented in a logical sequence, building upon previous knowledge and paving the way for a smooth progression of learning. The use of headings, subheadings, and bullet points makes it easy for students to navigate through the content and locate specific information. Overall, our Chemistry Form 1-4 Notes provide a comprehensive and well-structured resource for students studying chemistry. With its clear explanations, practical examples, and emphasis on safety, this resource is sure to enhance students' understanding and appreciation of the subject. Comprehensive Chemistry Summary Notes for Form 1 to Form 4Review: The "FORM 1 TO FORM 4 CHEMISTRY SUMMARY NOTES.pdf" is an invaluable resource for students studying chemistry at the Form 1 to Form 4 level. The notes provide a comprehensive overview of the entire chemistry syllabus, covering all the key topics and concepts. The document is well-organized, with each topic clearly outlined and explained in a concise manner. The content is presented in a way that is easy to understand, making it suitable for students of all levels of ability. The notes include detailed explanations, diagrams, and examples to help students grasp the fundamental principles of chemistry. One of the standout features of these notes is the inclusion of practical applications and real-life examples. This helps students to see the relevance of chemistry in everyday life and enhances their understanding of the subject. The document also includes a section on exam tips and strategies, which is particularly helpful for students preparing for their chemistry exams. It provides guidance on how to approach different types of questions, how to structure answers, and how to effectively revise for exams. Overall, the "FORM 1 TO FORM 4 CHEMISTRY SUMMARY NOTES.pdf" is a comprehensive and well-structured resource that covers all the essential topics in chemistry. Whether you are a student looking for additional study material or a teacher seeking a reference guide, these notes are a valuable asset.
Explain why commercial indicators are preferred to flower exacts as acid-base indicators.
ANSWERS
Explain why it is important to put off a non-luminous flame immediately after use.
ANSWERS
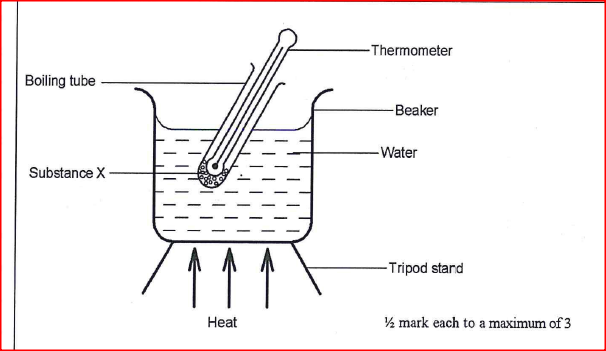
You are provided with the following: thermometer, boiling tube, beaker, Bunsen burner, pure substance X whose boiling point is about 80°C, water and any other apparatus that may be required. Draw a labelled diagram of the set-up that can be used to determine the melting point of X.
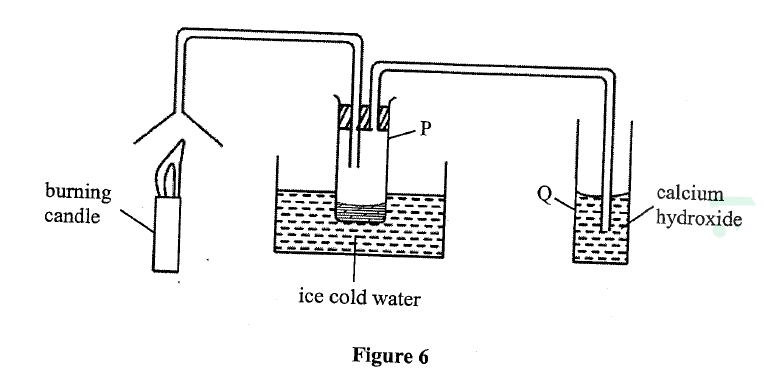
Study the set-up in Figure 6 and answer the questions that follow.
(a) Name the substance that was collected in tube p.
(b) Write an equation for the reaction which occurs in tube Q in the first few minutes of the experiment. (c) Give a suitable Conclusion for the experiment in the set-up.
Using iron filings, describe an experiment that can be conducted to show that oxygen is present in air.
ANSWERS
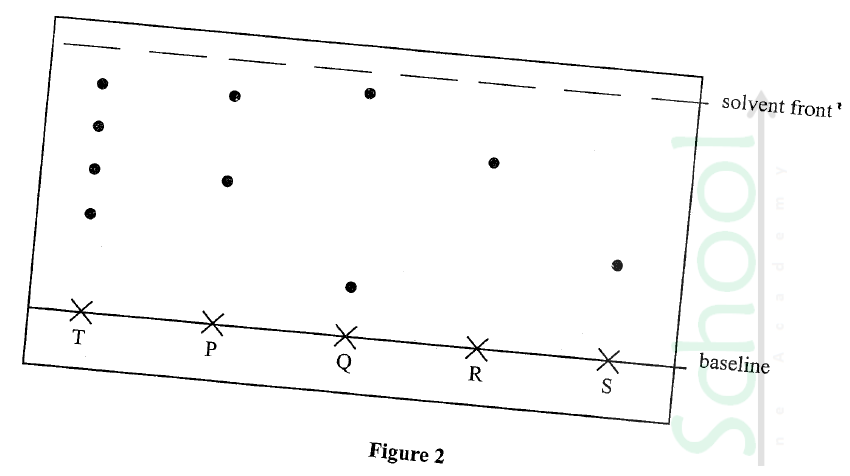
An experiment was Carried out to determine the presence of Substances P, Q, R and S in mixture T. The results obtained are shown in Figure 2.
(a) Ne the method of separation illustrated in Figure 2.
(b) Select: (i) one Substance Which Contains a Component not present in T. (ii) a substance which is least Soluble in the solvent used.
ANSWERS
(a)Chromatography/paper chromatography
(b)(i)Q (ii)S
Describe an experiment to show that group one elements react with cold water to form alkaline Solutions
ANSWERS
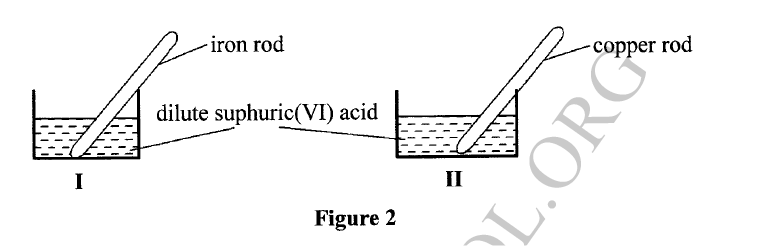
(a) A student used Figure 2 to investigate the action of dilute sulphuric(VI) acid on some metals. Beaker I and II contained equal volumes of dilute sulphuric(VI) acid. To beaker I, a clean iron rod was dipped and to beaker II, a clean copper rod was dipped.
(i) Why was it necessary to clean the metal rods?
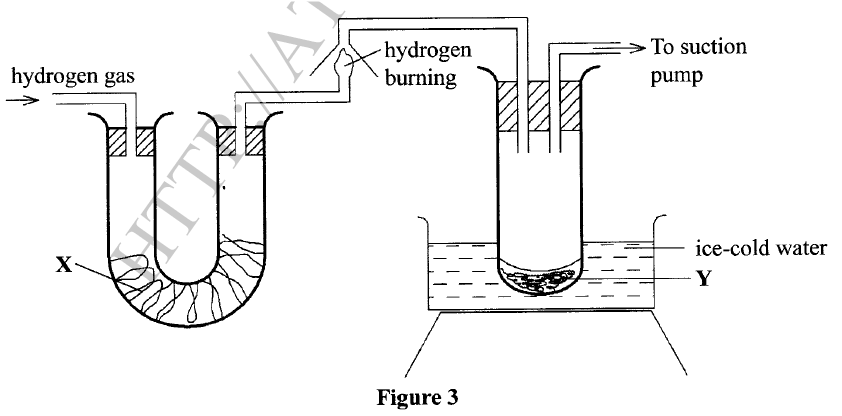
(ii) Describe the observations made in each beaker. Beaker I: Beaker II: (iii) Explain the observations in (a) (ii). (b) Figure 3 shows the apparatus used to burn hydrogen in air. Use it to answer the questions that follow.
(i)State the role of substance X.
(ii) Give the name of the substance that could be used as X. (iii) State the role of the suction pump. (iv) Name the product Y formed. (v) Give a simple physical test to prove the identity of Y. vi) State the difference between 'dry' and 'anhydrous'.
ANSWERS
(a) (i) To remove oxide layer on the metal.
(ii) Beaker I : . Bubbles of a colourless gas / effervescence ; . Solution turns green; . the size of iron rod decreases Beaker II: . The solution remained colourless. . No bubbles/effervescence
OR
Iron is more reactive than hydrogen hence it reacts with sulphuric(VI) acid to produce hydrogen gas and iron(III) sulphate which is green. Beaker II: Copper is below hydrogen hence no reaction will take place. (b) (i) To dry hydrogen gas. (ii) Calcium oxide /anhydrous calcium chloride /silica gel.
Explain how a student can establish whether a liquid sample extracted from a plant is pure.
ANSWERS
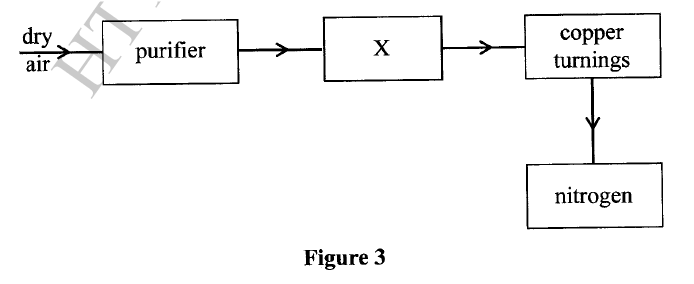
The flow chart in Figure 3 shows the process of obtaining a sample of nitrogen gas. Study it and answer the questions that follow.
(a) Identify X
(b) Write an equation for the reaction with heated copper turnings. (c) Name an impurity in the sample of nitrogen gas.
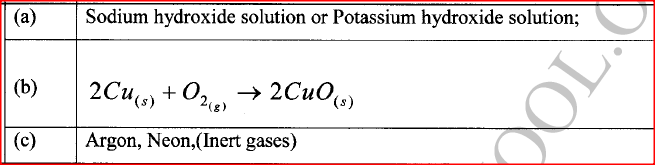
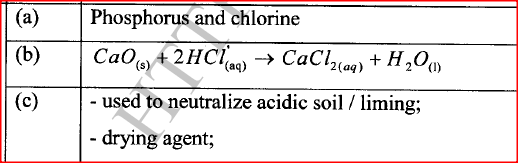
Using the elements chlorine, calcium and phosphorus:
(a) Select elements that will form an oxide whose aqueous solution has a pH less than 7. (b) Write an equation for the reaction between calcium oxide and dilute hydrochloric acid. (c) Give one use of calcium oxide.
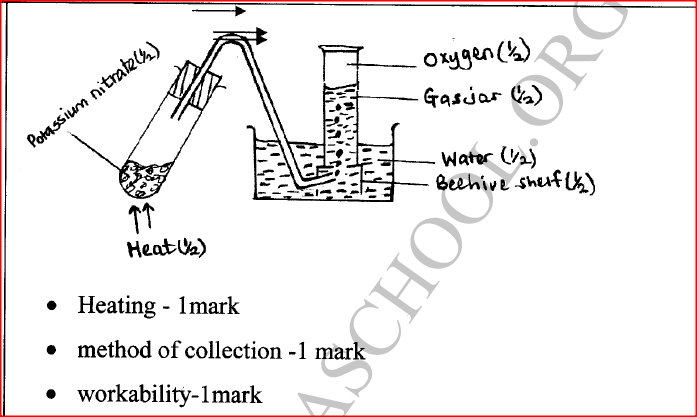
Potassium nitrate liberates oxygen gas when heated. Draw a diagram of a set-up that shows heating of potassium nitrate and collection of oxygen gas.
When a student was stung by a nettle plant, a teacher applied an aqueous solution of ammonia to the affected area of the skin and the
student was relieved of pain. Explain.
ANSWERS
Describe an experimental procedure that can be used to extract oil from nut seeds.
ANSWERS
Crush grind using a pestle and mortar, add suitable solvent of propanone ethanol alcohol and stir to dissolve oil. Filter the mixture to obtain a solution of the oil. Leave the solution in the sun for propanone to evaporate leaving the oil.
Given the following substances: wood ash, lemon juice and sodium chloride.
(a) Name one commercial indicator that can be used to show whether wood ash, lemon juice and sodium chloride are acidic, basic or neutral. (b) Classify the substances in 15(a) above as acids, bases or neutral.
ANSWERS
(a) Litmus
Phenolphthalein indicator (b) Wood ash- Basic Lemon Juice - acidic Sodium Chloride- neutral
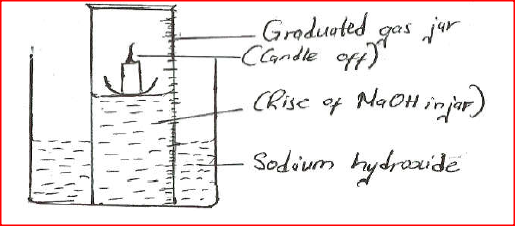
A water trough, aqueous sodium hydroxide, burning candle, watch class and a graduated gas jar were used in an experimental set up to determine the percentage of active part of air. Draw a labeled diagram of the set up at the end of the experiment.
Name an appropriate apparatus that is used to prepare standard solutions in the laboratory. (1 mark)
Expected Response
volumetric flask
Related Chemistry Questions and Answers on Simple Classification of Substances Form 1 Level
Iron (III) oxide was found to be contaminated with copper (II) sulphate. Describe how a pure sample of iron (III) oxide can be obtained.
ANSWERS
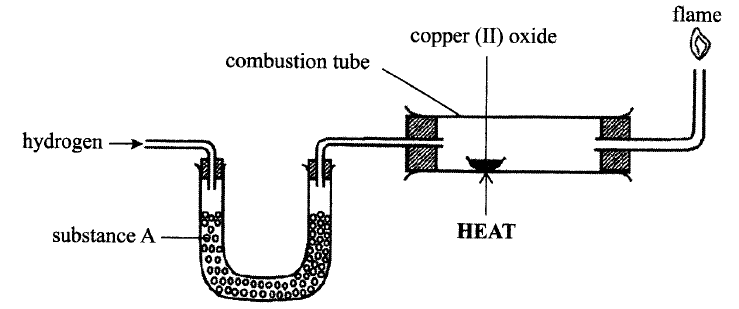
The set up below was used to investigate the reaction between dry hydrogen gas and copper
(a) Name substance A.
(b) State the observation made in the combustion tube. (c) Explain the observation stated in (b) above.
ANSWERS
(a) Fused anhydrous calcium chloride
Cao: fused CaCl2 (b) Black CuO changes to brown Cu metal Formation of colourless liquid on the cooler parts of the combustion tube. (c) Copper (II) oxide is reduced by hydrogen to copper metal while hydrogen is oxidized to water /CuO reduced to Cu /H2 Oxidized to H2O
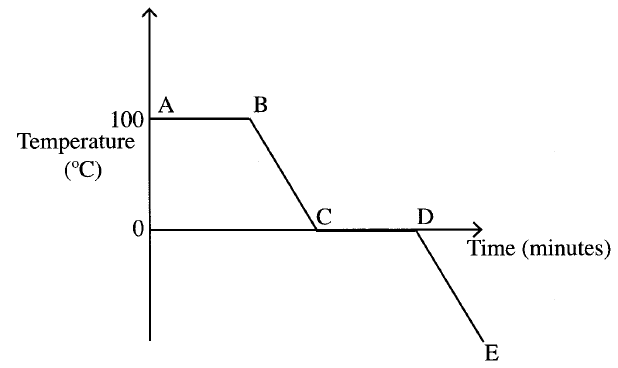
The graph below is a cooling curve for water. Study it and answer the questions that follow
(a) Explain what happens to the molecules of water in the region BC in terms of kinetic theory.
(b) In what state is the water in the region DE?
ANSWERS
The molecules of water are
(a) Loosing heat . The kinetic energy decreases and the molecules move closer to each other (b) Solid state |
Chemistry Topics
All
Archives
December 2024
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
|
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |






 RSS Feed
RSS Feed

