|
(a) Write an equation to show the effect of heat on the nitrate of:
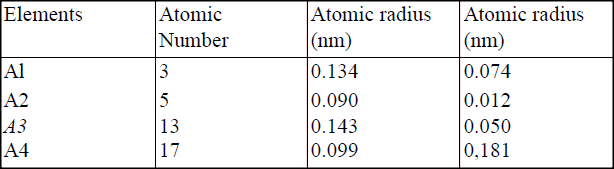
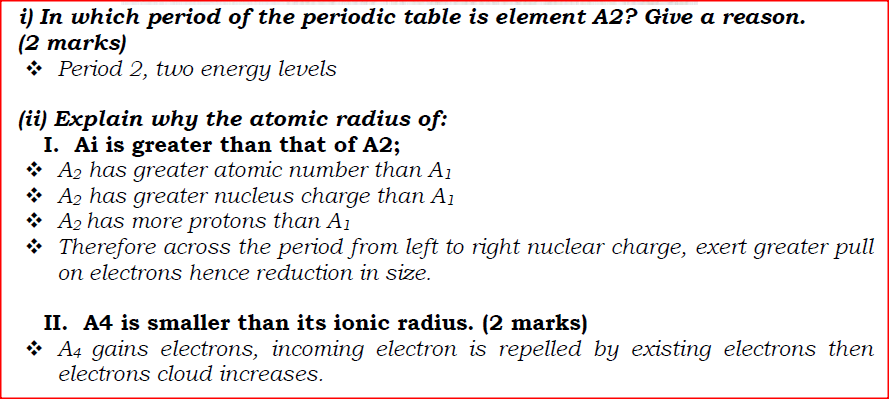
(i) Potassium (ii) Silver (b) The table below gives information about elements Ai, A2, A3, and A4 i) In which period of the periodic table is element A2? Give a reason.
(ii) Explain why the atomic radius of:
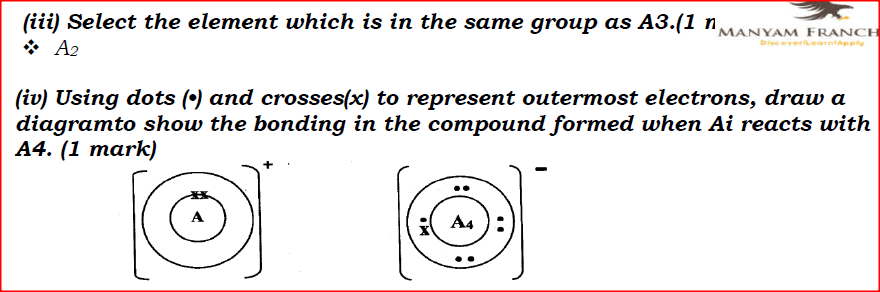
I. Ai is greater than that of A2; II. A4 is smaller than its ionic radius. (iii) Select the element which is in the same group as A3. (iv) Using dots (•) and crosses(x) to represent outermost electrons, draw a diagramto show the bonding in the compound formed when Ai reacts with A4.
0 Comments
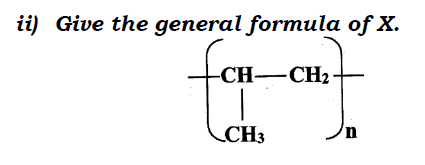
a)Draw the structural formula for all the isomers of C2H3CL3
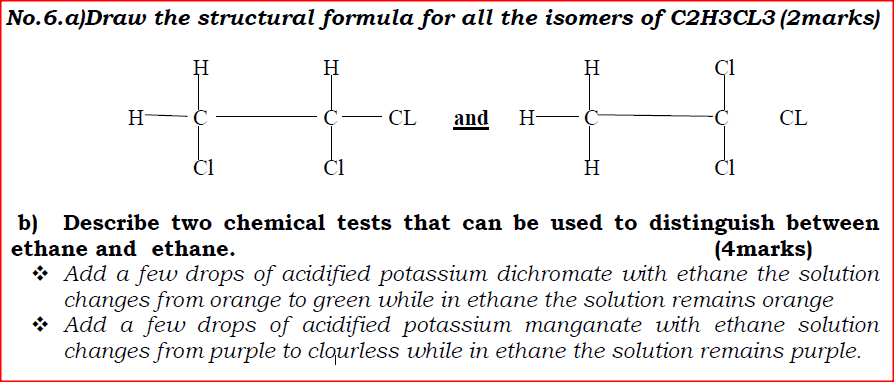
b) Describe two chemical tests that can be used to distinguish between ethane and ethane.
c)The following scheme represents various reactions starting with propan-1-ol. Use it to answer the questions that follow.
i) Name one substance that can be used in step I.
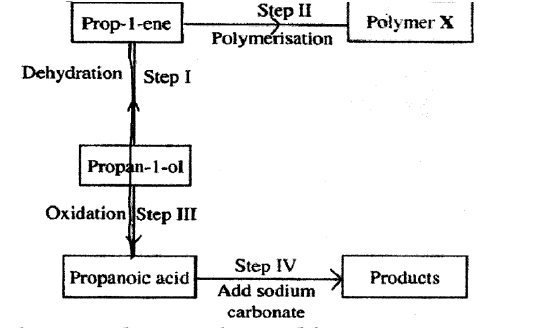
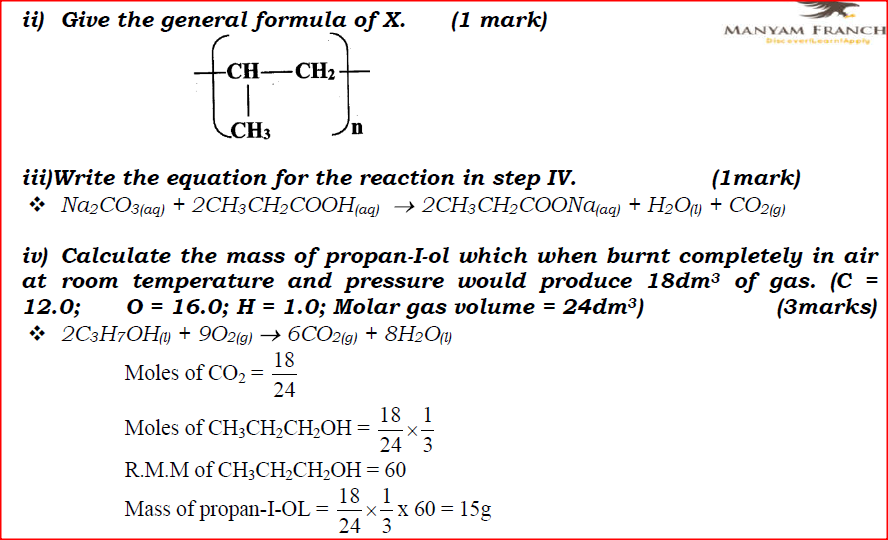
ii) Give the general formula of X.
iii)Write the equation for the reaction in step IV.
iv) Calculate the mass of propan-1-ol which when burnt completely in air at room temperature and pressure would produce 18dm3 of gas. (C = 12.0; O = 16.0; H = 1.0; Molar gas volume = 24dm3)
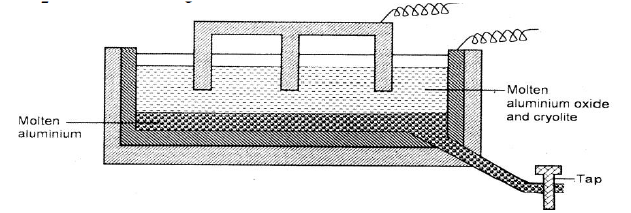
The diagram below represents a set up of an electrolytic cell that can be used in the production of aluminium.
(a) On the diagram, label the anode.
(b)Write the equation for the reaction at the anode. (c) Give a reason why the electrolytic process is not carried out below 950°C. (d)Give a reason why the production of aluminium is not carried out using reduction process (e)Give two reasons why only the aluminium ions are discharged (f)State two properties of duralumin that makes it suitable for use in aircraft industry. (g)Name two environmental effects caused by extraction of aluminium.
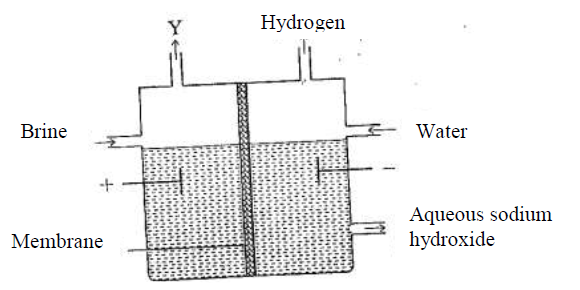
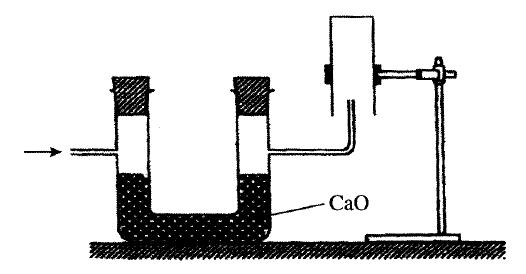
The set up below can be used to produce sodium hydroxide by electolysing brine.
(i) Identify gas Y.
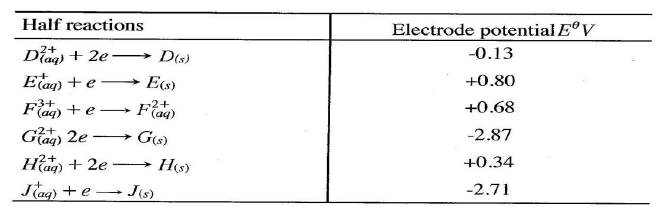
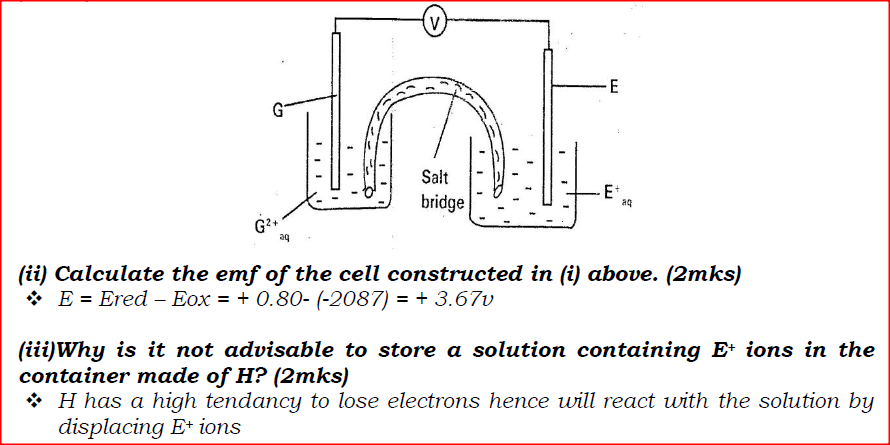
(ii)Describe how aqueous sodium hydroxide is formed in setup above. (iii)One of the uses of sodium hydroxide is in manufacture of soaps. State one other use of sodium hydroxide. (b) Study the information given in the table below and answer the questions that follow.
(i) Construct an electrochemical cell that will produce the highest
(ii) Calculate the emf of the cell constructed in (i) above. (iii)Why is it not advisable to store a solution containing E+ ions in the container made of H?
(a) Methanol is manufactured from carbon (IV) oxide and hydrogen gas according to the equation:
The reaction is carried out in the presence of a chromium catalyst at 700K and 30kPa. Under these conditions, equilibrium is reached when 2% of the carbon (IV) oxide is converted to methanol
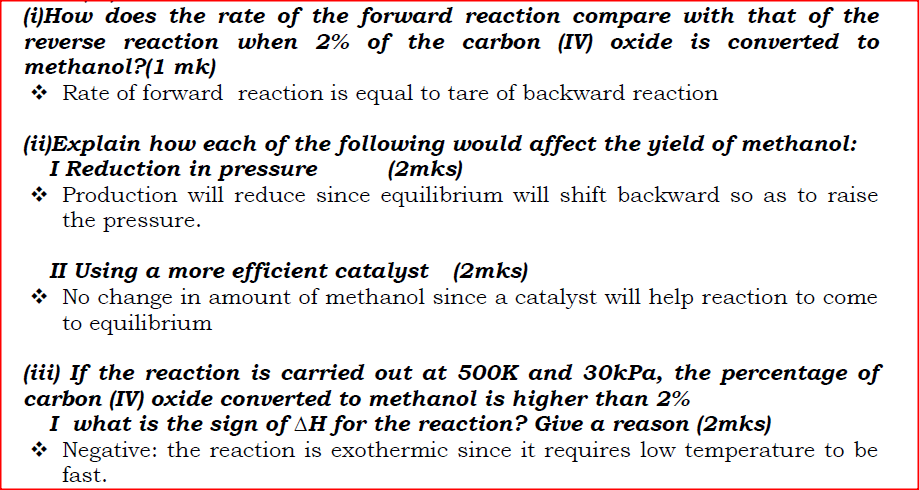
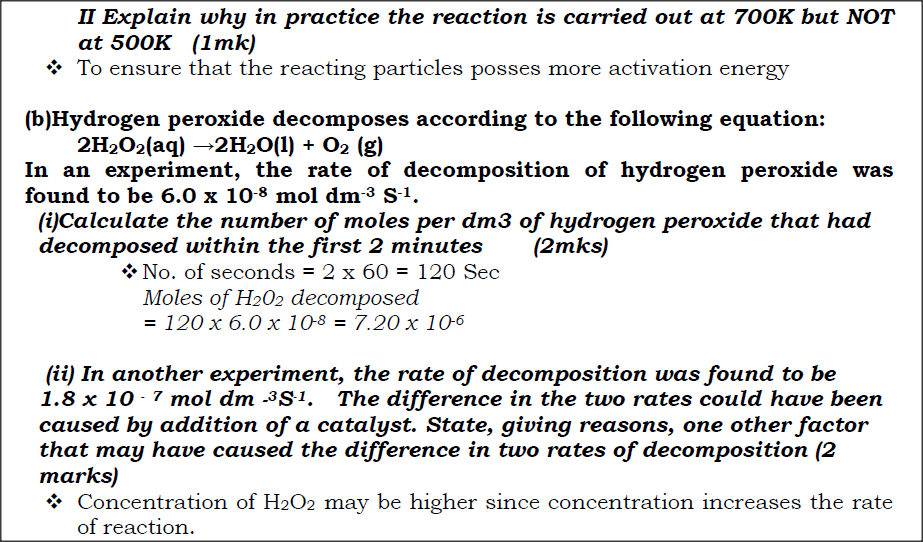
(i)How does the rate of the forward reaction compare with that of the reverse reaction when 2% of the carbon (IV) oxide is converted to methanol? (ii)Explain how each of the following would affect the yield of methanol: I Reduction in pressure II Using a more efficient catalyst (iii) If the reaction is carried out at 500K and 30kPa, the percentage of carbon (IV) oxide converted to methanol is higher than 2% I what is the sign of ΔH for the reaction? Give a reason II Explain why in practice the reaction is carried out at 700K but NOT at 500K (b)Hydrogen peroxide decomposes according to the following equation:
2H2O2(aq) →2H2O(l) + O2 (g)
In an experiment, the rate of decomposition of hydrogen peroxide was found to be 6.0 x 10-8 mol dm-3 S-1. (i)Calculate the number of moles per dm3 of hydrogen peroxide that had decomposed within the first 2 minutes (ii) In another experiment, the rate of decomposition was found to be 1.8 x 10 - 7 mol dm -3S-1. The difference in the two rates could have been caused by addition of a catalyst. State, giving reasons, one other factor that may have caused the difference in two rates of decomposition
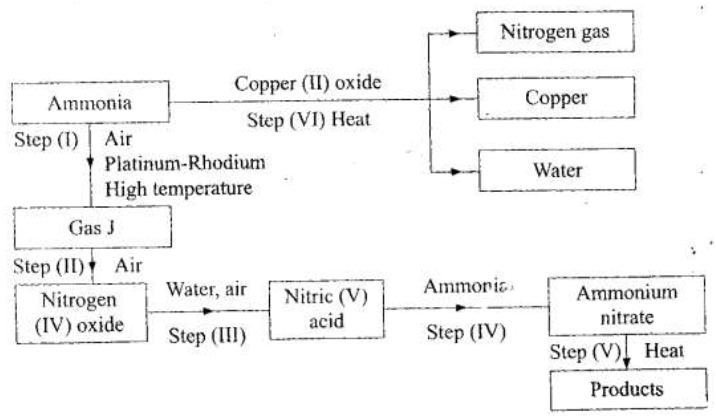
(a) Describe the process by which Nitrogen is obtained from air on a large scale.
(b) Study the flow chart below and answer the questions that follow.
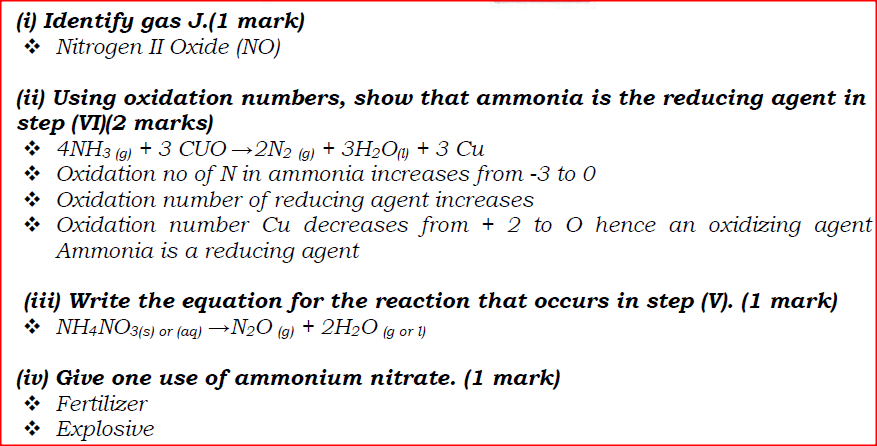
(i) Identify gas J.
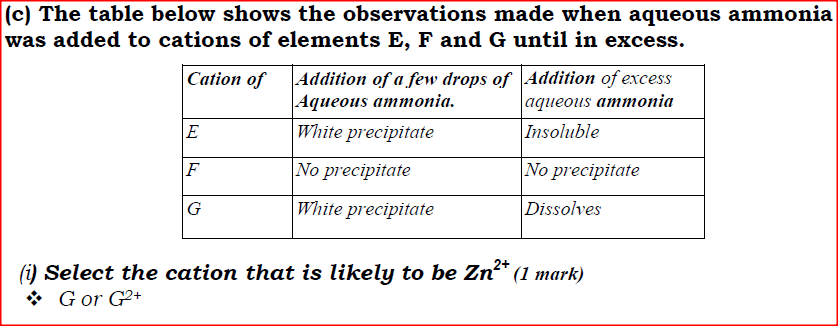
(ii) Using oxidation numbers, show that ammonia is the reducing agent in step (VI) (iii) Write the equation for the reaction that occurs in step (V). (iv) Give one use of ammonium nitrate. (c) The table below shows the observations made when aqueous ammonia was added to cations of elements E, F and G until in excess.
(i) Select the cation that is likely to be Zn2+
(ii) Given that the formula of the cation of element E is E 2+ , write the ionic equation for the reaction between E2+(aq) and aqueous ammonia.
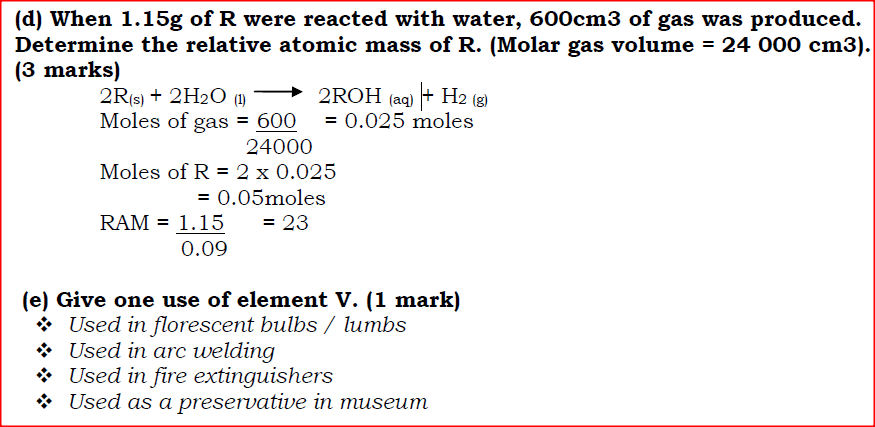
Use the information in the table below to answer the questions that follow. The letters do not represent the actual symbols of the elements.
(a) Give reasons why the melting point of:
(i) S is higher than that of R; (ii) V is lower than that of U. (b) How does the reactivity of W with Chlorine compare with that of R with chlorine? Explain. (c) Write an equation for the reaction between T and excess oxygen. (d) When 1.15g of R were reacted with water, 600cm3 of gas was produced. Determine the relative atomic mass of R. (Molar gas volume = 24 000 cm3). (e) Give one use of element V.
When a student was stung by a nettle plant, a teacher applied an aqueous solution of ammonia to the affected area of the skin and the
student was relieved of pain. Explain.
ANSWERS
A mixture contains ammonium chloride, copper (II) oxide and sodium chloride. Describe how each of the substances can be obtained form the mixture.
ANSWERS
Describe an experimental procedure that can be used to extract oil from nut seeds.
ANSWERS
Crush grind using a pestle and mortar, add suitable solvent of propanone ethanol alcohol and stir to dissolve oil. Filter the mixture to obtain a solution of the oil. Leave the solution in the sun for propanone to evaporate leaving the oil.
Hydrogen sulphide is a highly toxic and flammable gas. It is normally prepared in a fume chamber.
(a) Name two reagents that can be used to prepare hydrogen sulphide in the laboratory. (b) One of the uses of hydrogen sulphide is to produce sulphur as shown in the following equation: 2H2S(g) + S02 (g) -> 3S(s) + 2H20(1) Identify the reducing agent in this reaction and give a reason for your answer. (c) Other than production of sulphuric (VI) acid, state one commercial use of sulphur.
ANSWERS
(a)Iron (II) sulphide or conc sulphide / copper sulphide (Accp formula: Fes/ HCl)
Hydrochloric acid or lead (II) sulphide/ HNO3 (b)Hydrogen sulphide The sulphur changes from -2 to zero/ (it reduces SO2 to S) i.e. +4 to 0 / sulphur lost e’s in the H2S to form sulphur (c)Vulcanization of rubber Manufacture of sulphur drugs Manufacture of gun powder/ match sticks / explosives/ fungicides
Charcoal is a fuel that is commonly used for cooking. When it burns it forms two oxides.
(a) Name the two oxides. (b) State one use of the two oxides.
ANSWERS
(a)Carbon (IV) oxide /CO2/ carbon dioxide
Carbon (II) oxide/ CO/ carbon monoxide (b)Fire extinguisher/ photosynthesis Refrigeration Solvay process Fizzy drinks Food preservation Extraction of metals Manufacture of methanol Manufacture of fuel (water, gas)
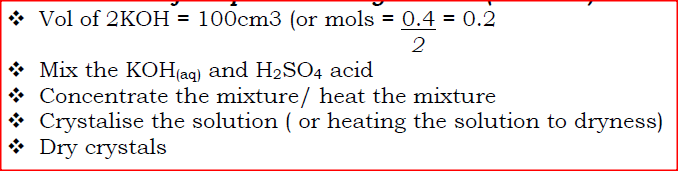
Describe how a solid sample of potassium sulphate can be prepared starting with 200cm3of 2M potassium hydroxide.
Given the following substances: wood ash, lemon juice and sodium chloride.
(a) Name one commercial indicator that can be used to show whether wood ash, lemon juice and sodium chloride are acidic, basic or neutral. (b) Classify the substances in 15(a) above as acids, bases or neutral.
ANSWERS
(a) Litmus
Phenolphthalein indicator (b) Wood ash- Basic Lemon Juice - acidic Sodium Chloride- neutral
The set-up below was used to collect a dry sample of a gas
Give two reasons why the set-up cannot be used to collect carbon (IV) oxide gas.
ANSWERS
Draw and name the isomers of pentane.
In an experiment on rates of reaction, potassium carbonate was reacted with dilute sulphuric (VI) acid.
(a) What would be the effect of an increase in the concentration of the acid on the rate of the reaction? (b) Explain why the rate of reaction is found to increase with temperature.
ANSWERS
(a)The rate of reaction increases. This is because when the concentration is high: the number of collisions between particles is also high hence reacts faster,
(b)Increase in temperature results in increase in the kinetic energy of the particles. This makes particles move faster and collide frequently leading to faster rate of reaction.
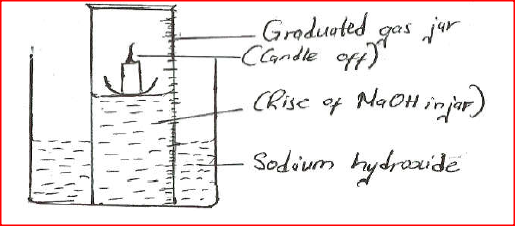
A water trough, aqueous sodium hydroxide, burning candle, watch class and a graduated gas jar were used in an experimental set up to determine the percentage of active part of air. Draw a labeled diagram of the set up at the end of the experiment.
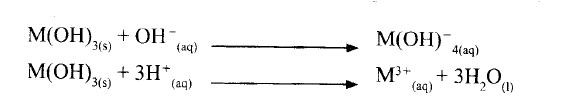
A compound whose general formula is M(OH)3 reacts as shown by the equation below.
(a) What name is given to compounds which behave like M(OH) 3 in the two reactions.
(b) Name two elements whose hydroxides behave like that of M.
ANSWERS
(a) Amphoteric
(b)Lead, Zinc and Aluminium
The atomic number of sulphur is 16. Write the electron arrangement of sulphur in the following:
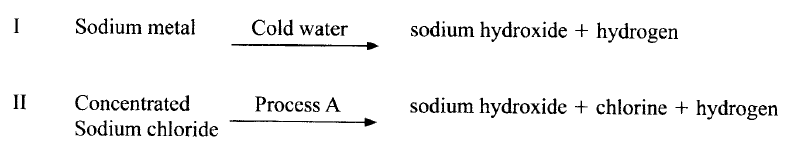
Sodium hydroxide can be prepared by the following methods; I and II
(a) Name one precaution that needs to be taken in method I.
(b) Give the name of process A. (c) Give one use of sodium hydroxide.
ANSWERS
(a) Small piece of sodium metal (pea size) with a lot of water
Perform the experiment wearing goggles. (b) Electrolysis (c) Manufacture of paper (soften), soaps and detergents Fractional distillation of liquid air Extraction of aluminium metal Manufacture of bleaching agents eg NaOCl paper, textiles, oil refinery Making herbicides on weed killers Textile industry to soften
When fuels burn in the internal combustion engine at high temperature, one of the products formed is nitrogen (II) oxide.
(a) Write the equation for the formation of nitrogen (II) oxide. (b) Give a reason why nitrogen (II) oxide is not formed at room temperature. (c) Describe how formation of nitrogen (II) oxide in the internal combustion engine leads to gaseous pollution.
ANSWERS
(a)N2(g) + 02(g) 2NO(g)
(b)Nitrogen atoms in the molecule are joined by strong triple covalent bond that requires a lot of energy to break than provided at room temperature (c) Nitrogen (II) oxide reacts with oxygen in air to form nitrogen (IV) oxide that dissolves in water vapour causing acid rain.
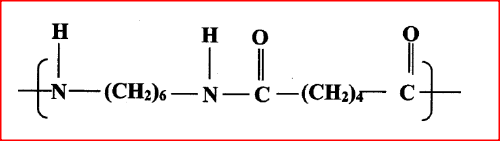
(a)Draw the structure of compound N formed in the following reaction.
(b) Give one use of compound N.
During an experiment, chlorine gas was bubbled into a solution of potassium iodide.
(a)State the observations made. (b)Using an ionic equation, explain why the reaction is redox
ANSWERS
(a)Solution turned from colourless to dark brown
Greenish yellow / pale green colour of Cl2 disappears Brown solution / black solid is deposited (b) Cl2 (aq) +2I – (aq) → I2 (aq)+2CI- (aq) Explanation; Iodine oxidation state changes from -1 to 0 hence oxidation while Cl2 0.5 changes from 0 to -1 hence reduction / increase is ON and decrease is ON or movement of electrons Cl2 gains e’s where lose
a)Complete the nuclear equation below:
(c)Give one harmful effect of radioisotopes.
|
Chemistry Topics
All
Archives
December 2024
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
|
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |
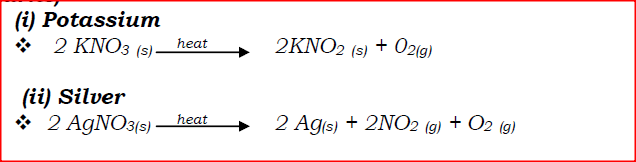



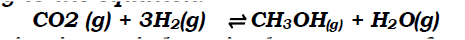






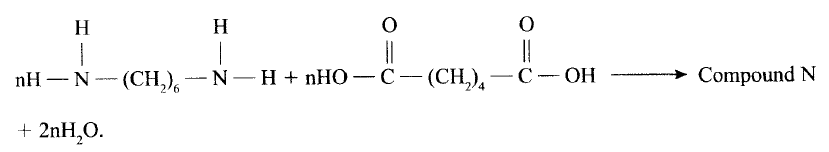

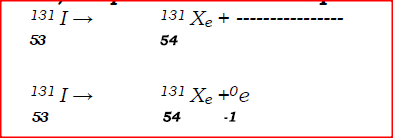

 RSS Feed
RSS Feed

