KCSE CHEMISTRY QUESTIONS AND ANSWERS PER TOPIC
|
ANSWERS
0 Comments
ANSWERS
a)Dilute Nitric acid
b)Silver metal c)oxygen
In an experiment, a few drops of concentrated nitric acid were added to aqueous iron(II) sulphate in a test – tube. Excess sodium hydroxide solution was then added to the mixture.
a) State the observations that were made when: i) Concentrated nitric acid was added to aqueous iron (II) sulphate ii) Excess sodium hydroxide was added to the mixture. b) Write and ionic equation for the reaction which occurred in (a) (ii) above
When a student was stung by a nettle plant, a teacher applied an aqueous solution of ammonia to the affected area of the skin and the student was relieved of pain .Explain.
ANSWERS
ANSWERS
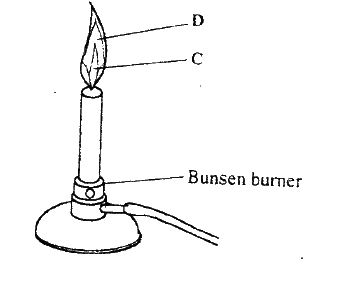
C- unburnt gas
D- Luminous yellow flame
Both chlorine and iodine are halogens.
a) What are halogens? b) In terms of structure and bonding, explain why the boiling point of chlorine is lower than that of iodine.
ANSWERS
(a) Group (VIII) elements
(b) Chlorine molecule is smaller and the strength of Vander Waals forces between molecules of chlorine is weak as compared to iodine.
15.0cm3 of ethanoic acid (CH3COOH) was dissolved in water to make 500cm3 of solution. Calculate the concentration of the solution in moles per litre. (C=12.0;H=1.0;O=16.0; density of ethanoic acid is 1.05 g/cm3)
ANSWERS
(a) Carbon (IV) oxide
(b) Blue flame, carbon (II) oxide is burning |
Chemistry Topics
All
Archives
December 2024
|
We Would Love to Have You Visit Soon! |
Hours24 HR Service
|
Telephone0728 450425
|
|
8-4-4 materialsLevels
Subjects
|
cbc materialsE.C.D.E
Lower Primary
Upper Primary
Lower Secondary
Upper Secondary
|
teacher support
Other Blogs
|








 RSS Feed
RSS Feed