|
These are chemistry questions and answers categorized according to topics, papers i.e. Paper 1 and 2, Levels i.e. form 1 to form 4, kcse year the examination was done and section A or B
Select topic/category to open topical questions from that particular option provided. Chemistry Topics
0 Comments
The cell convention for an electrochemical cell is shown belowa) Name two substances that can be used as electrolytes in the above cell (2mks)
Zinc sulphate or any other solution with zinc ions
b) Which of the electrodes is the anode? (2mks)
Lead electrode
When an electric current was passed through molten substances M and N in different containers the observations in the table below were made
Suggest the type of bonding present in;a) Substance M (1mk)
Metallic bond
b) Substance N (1mk)
Ionic bond
Explain why a solution of sodium chloride Conducts electricity while that of sugar does not.
ANSWERS
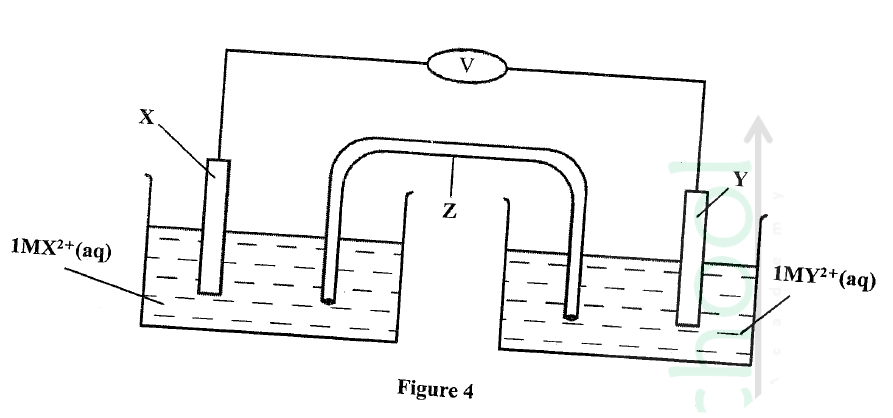
Metal X and Y have standard electrode Potentials of —0.13 V and —0.76V respectively .The metals were Connected to form a cell as shown in Figure 4.
(a) Name the part labelled z.
(b) State one functi0 of the part labelled Z. (c) Calculate the e.m.f. of the cell.
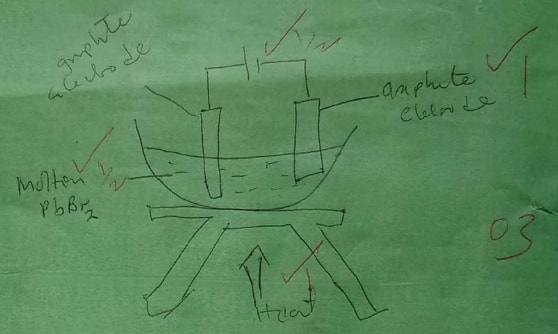
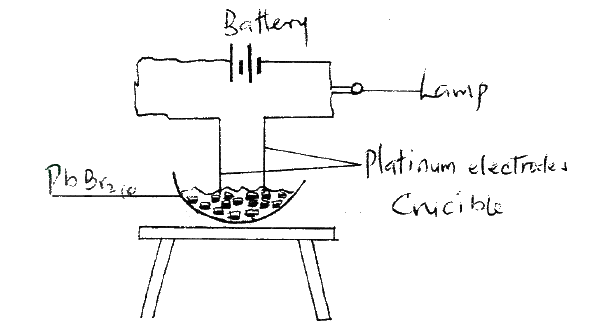
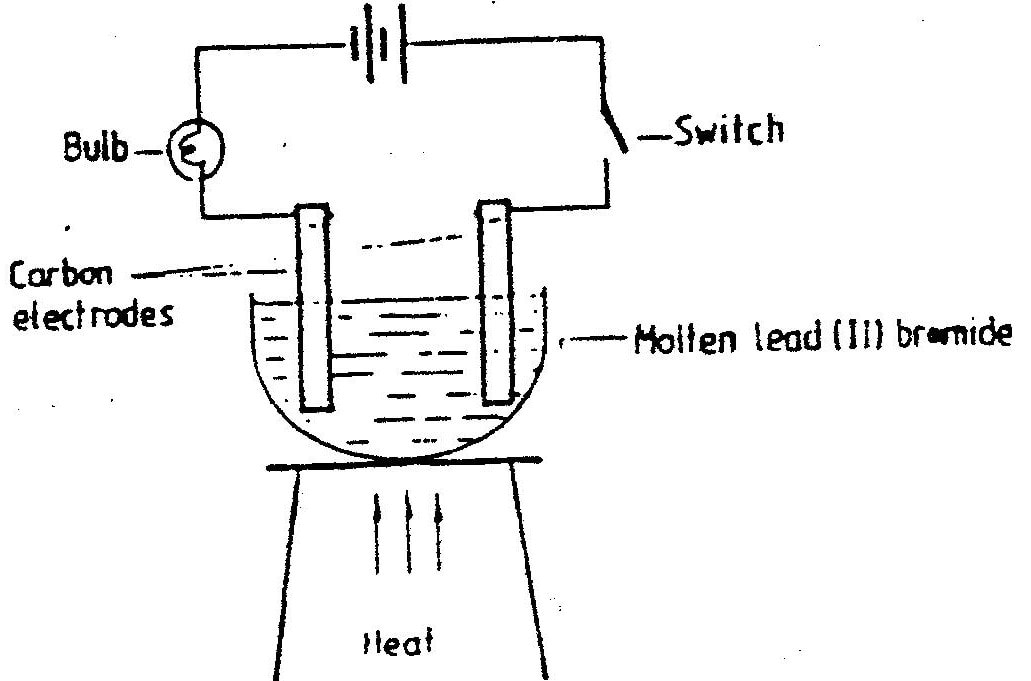
Draw in the space provided a labelled diagram of the set-up of the apparatus that can be used to electrolyse molten Lead (II) bromide. (3 marks)
Related Chemistry Questions and Answers on Effects of Electric Current on Substances Form 2 Level
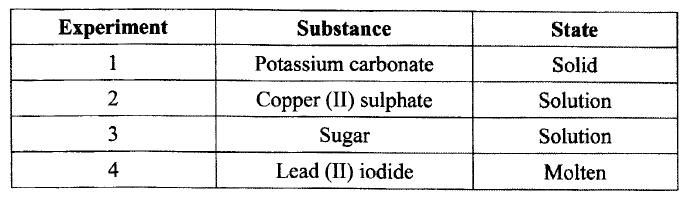
A student investigated the effect of an electric current by passing it through some substances. The student used inert electrodes, and connected a bulb to the circuit. The table below shows the substances used and their
states.
(a) In which experiment did the bulb not light?
(b) Explain your answer in (a) above.
ANSWERS
(a) 1 and 3
(b)In 1 ions K2CO3 are held rigidly within the crystal cannot move (no mobile ions) In 3 sugar exist as molecule hence no mobile ions.
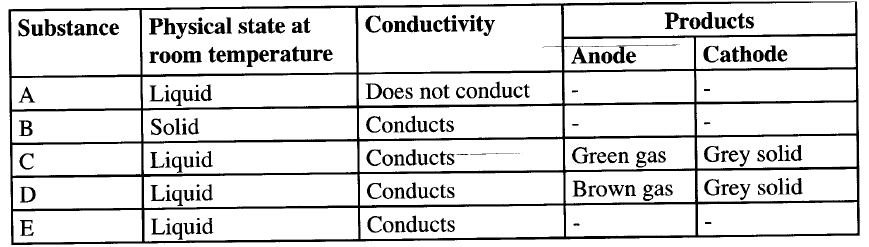
An electric current was passed through several substances and the results obtained recorded in the table below.
Which of these substance is likely to be
a)Magnesium b) Hexane c) lead (II) bromide ?
ANSWERS
Explain how condition of electricity takes place in the following.
(a) Iron metal (b) Molten lead (II) iodide
ANSWERS
(a) delocalised electrons.
(b) Ions in the melt.
A student investigated the effect of an electric current by passing it through some substances.
The student used inert electrodes, and connected a bulb to the circuit. The table below shows the substances used and their states.
a) In which experiments did the bulb not light?
b) Explain your answer in (a) above.
Charcoal is a fuel that is commonly used for cooking. When it burns it forms two oxides.
(a) Name the two oxides. (b) State one use of any of the two oxides.
ANSWERS
(a) Carbon (IV) Oxide and Carbon (II) Oxide
(b) CO2- Fire extinguishers Fizzy drinks Food preservative . Solvay process . CO -Manufacture of fuel (water gas) Reduction in the extraction of metals Manufacture of methanol
Explain why the following substances conduct an electric current.
(a) Magnesium metal. (b) Molten magnesium chloride.
ANSWERS
Study the set – up below and answer the question that follows.
State and explain the observations that would be made when the circuit is completed.
|
Chemistry Topics
All
Archives
December 2024
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
|
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |
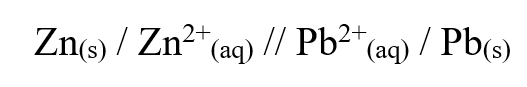









 RSS Feed
RSS Feed

