KCSE CHEMISTRY QUESTIONS AND ANSWERS PER TOPIC
|
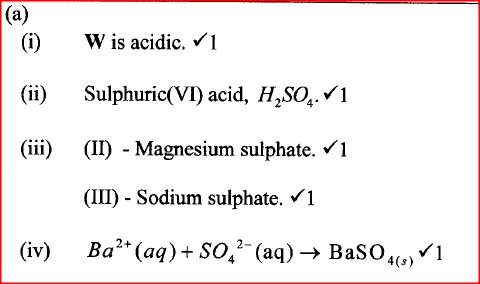
W is a colourless aqueous solution with the following properties:
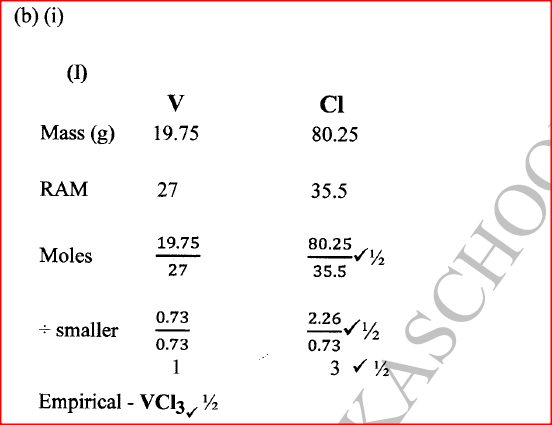
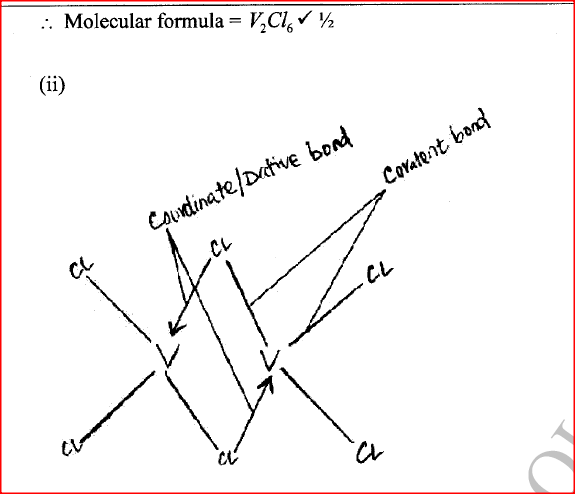
(ii) Give the identity of W. (iii) Name the colourless solution formed in (II) and (III). (iv) Write an ionic equation for the reaction indicated in (V). (b) Element V conducts electricity and melts at 933K. When chlorine gas is passed over heated V, it forms a vapour that solidifies on cooling. The solid chloride dissolves in water to form an acidic solution. The chloride vapour has a relative molecular mass of 267 and contains 19.75% of V. At a higher temperature, it dissociates to a compound of relative molecular mass 133.5. When aqueous sodium hydroxide is added to the aqueous solution of the chloride, a white precipitate is formed which dissolves in excess alkali. (V = 27.0 ; CI = 35.5) (i) Determine the:
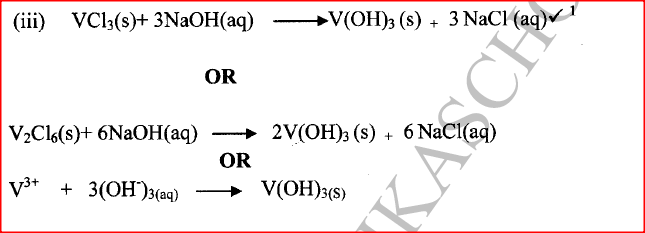
(iii) Write an equation for the reaction that form a white precipitate with sodium hydroxide. Related Chemistry Questions and Answers on Acids, Bases and Salts Form 4 Level
0 Comments
Leave a Reply. |
Chemistry Topics
All
Archives
December 2024
|
We Would Love to Have You Visit Soon! |
Hours24 HR Service
|
Telephone0728 450425
|
|
8-4-4 materialsLevels
Subjects
|
cbc materialsE.C.D.E
Lower Primary
Upper Primary
Lower Secondary
Upper Secondary
|
teacher support
Other Blogs
|
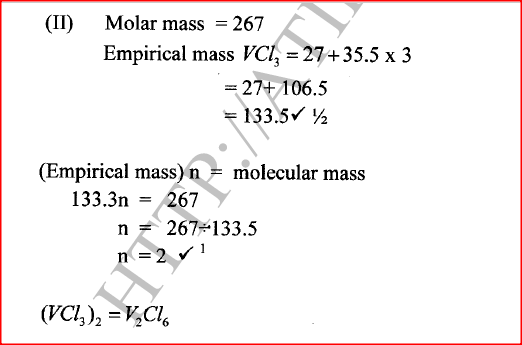

 RSS Feed
RSS Feed