|
The following steps were used to analyse a metal ore.
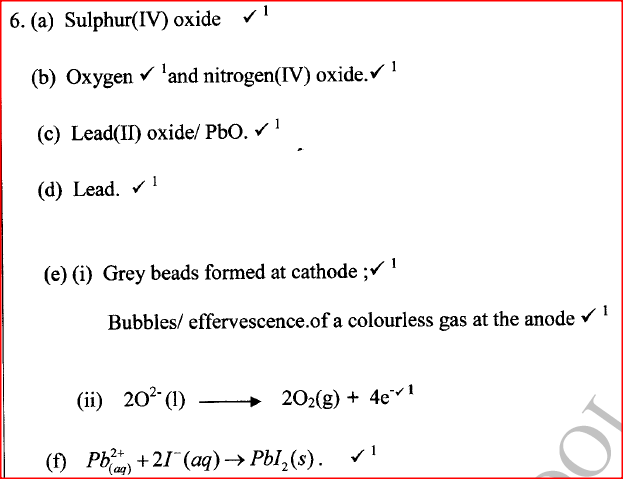
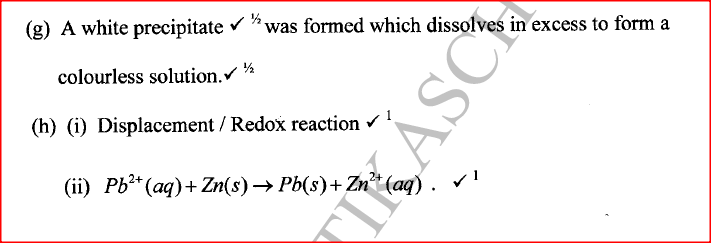
(i) An ore of a metal was roasted in a stream of oxygen. A gas with a pungent smell was formed which turned acidified potassium dichromate(VI) green. (ii) The residue left after roasting was dissolved in hot dilute nitric(V) acid. Crystals were obtained from the solution. (iii) Some crystals were dried and heated. A brown acidic gas and a colourless gas were evolved and a yellow solid remained. (iv) The solid was yellow when cold. (v) The yellow solid was heated with powered charcoal. Shiny beads were formed. Name the: (a) gas formed when the ore was roasted in air. (b) gases evolved when crystals in step (iii) were heated. (c) yellow solid formed in step (iii). (d) shiny beads in step (iv). (e) The yellow solid from procedure (iii) was separated, dried, melted and the melt electrolysed using graphite electrodes. I. Describe the observations made at each electrode. II. Write the equation for the reaction that took place at the anode. (f) Some crystals formed in step (ii) were dissolved in water, and a portion of it reacted with potassium iodide solution. A yellow precipitate was formed. Write an ionic equation for this reaction. (g) To another portion of the solution from (f), sodium hydroxide solution was added drop by drop until there was no further change. Describe the observation made. (h) To a further portion of the solution from (f), a piece of zinc foil was added. I. Name the type of reaction taking place. II. Write an ionic equation for the above reaction. Related Chemistry Questions and Answers on Metals Form 4 Level
0 Comments
Leave a Reply. |
Chemistry Topics
All
Archives
December 2024
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
|
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |

 RSS Feed
RSS Feed

