KCSE CHEMISTRY QUESTIONS AND ANSWERS PER TOPIC
|
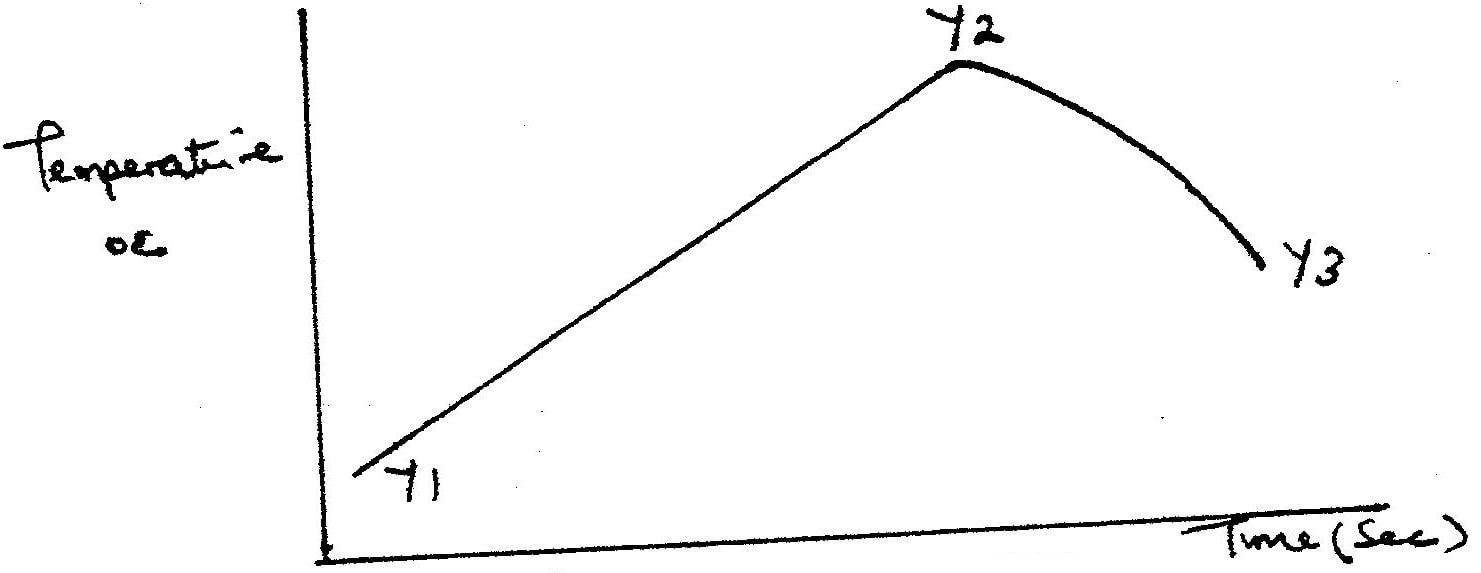
In order to determine the molar of neutralization of sodium hydroxide, 100cm3 of 1M sodium hydroxide and 100cm3 of 1 M hydrochloric acid both at the same initial temperature were mixed and stirred continuously with a thermometer. The thermometer of the resulting solution was recorded after every 30 seconds until the highest temperature of the solution was attained. Thereafter the temperature of the solution was recorded for a further two minutes
(a)
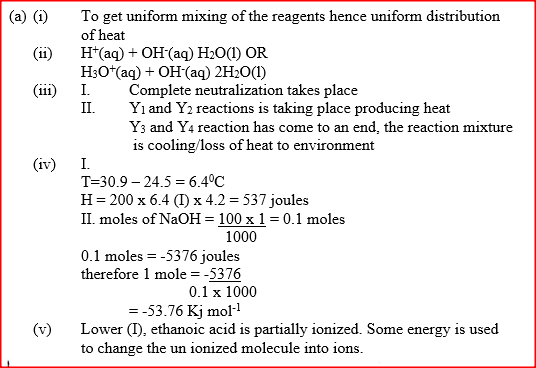
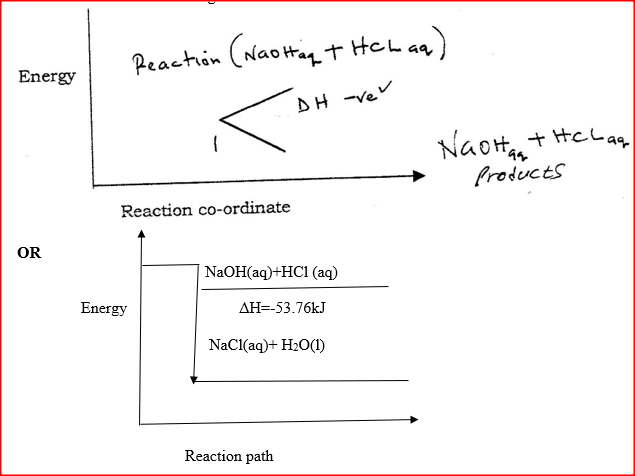
I. What is the significance of point Y2? II. Explain why there is a temperature change between points; Y1 and Y2 Y3 and Y4 (iv) In the initial temperature for both solutions was 24.50C and the highest temperature attained by the mixture was 30.90C Calculate the: I. heat change for the reaction (specific heat capacity of the solution = 4.2Jg -1K-1 and the density of the solution = 1.0g/cm3 II. Molar heat of neutralization of sodium hydroxide (v) Explain how the value of the molar heat of neutralization obtained in this experiment would compare with the one that would be obtained if the experiment was repeated using 100cm3 of 1 Methanoic acid instead of hydrochloric acid. (b) On the grid provided below, draw an energy level diagram for the reaction between hydrochloric acid and sodium hydroxide Related Chemistry Questions and Answers on Energy Changes in Chemical and Physical Processes Form 4 Level
0 Comments
Leave a Reply. |
Chemistry Topics
All
Archives
December 2024
|
We Would Love to Have You Visit Soon! |
Hours24 HR Service
|
Telephone0728 450425
|
|
8-4-4 materialsLevels
Subjects
|
cbc materialsE.C.D.E
Lower Primary
Upper Primary
Lower Secondary
Upper Secondary
|
teacher support
Other Blogs
|

 RSS Feed
RSS Feed