KCSE CHEMISTRY QUESTIONS AND ANSWERS PER TOPIC
|
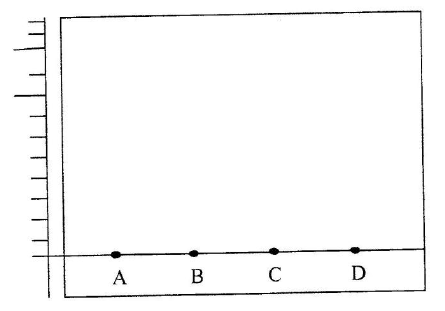
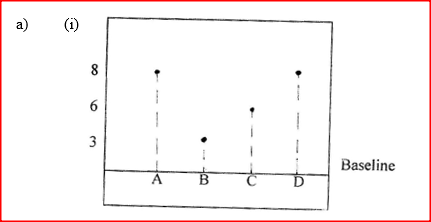
(a) The diagram below shows spots of pure substance A,B, and C on a chromatography paper. Spot D is that of a mixture
After development, A, B and C were found to have moved 8cm, 3cm and 6 cm respectively. D has separated into two spots which had moved 6cm and 8 cm
(i) On the diagram I Label the baseline ( origin) II Show the positions of all the spots after development (ii) Identify the substances present in the mixture D (b) Describe how solid ammonium chloride can be separated fro a solid mixture of ammonium chloride and anhydrous calcium chloride (c) The table shows liquids that are miscible and those that are immiscible
Use the information given to answer the questions that follow
(i) Name the method that can be used to separate L1 and L3 from a mixture of two (ii) Describe how a mixture of L2 and L4 can be separated
ANSWERS
(ii) A and C
b)Since NH4CL4 sublimes but CaCl2 does not; sublimation process would do. Heat the mixture. Ammonium chloride sublimates into vapour and condenses on the cooler part of the heating tube. Calcium chloride will remain on the bottom of the heating tube. c)i)Fractional distillation ii)Separating funnel method Since the tow liquids are immiscible, pour both the liquids in a separating funnel and allow to settle, the denser liquid will settle down and the less dense will form a second layer on top. Open the tape and run out the liquid in the bottom layer leaving the liquid in the second layer in the funnel. Related Chemistry Questions and Answers on Simple Classification of Substance Form 1 Level
0 Comments
Leave a Reply. |
Chemistry Topics
All
Archives
December 2024
|
We Would Love to Have You Visit Soon! |
Hours24 HR Service
|
Telephone0728 450425
|
|
8-4-4 materialsLevels
Subjects
|
cbc materialsE.C.D.E
Lower Primary
Upper Primary
Lower Secondary
Upper Secondary
|
teacher support
Other Blogs
|
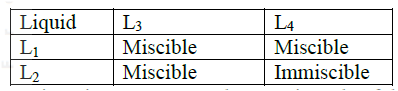

 RSS Feed
RSS Feed