|
These are chemistry questions and answers categorized according to topics, papers i.e. Paper 1 and 2, Levels i.e. form 1 to form 4, kcse year the examination was done and section A or B
Select topic/category to open topical questions from that particular option provided. Chemistry Topics
0 Comments
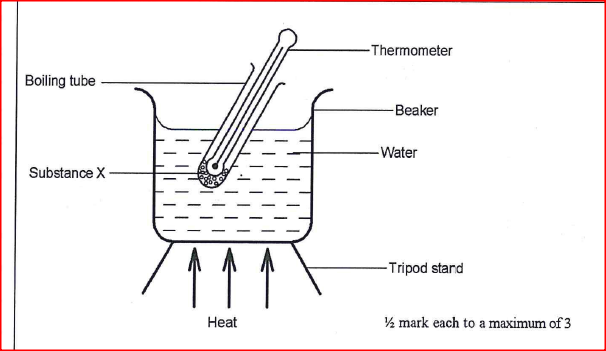
You are provided with the following: thermometer, boiling tube, beaker, Bunsen burner, pure substance X whose boiling point is about 80°C, water and any other apparatus that may be required. Draw a labelled diagram of the set-up that can be used to determine the melting point of X.
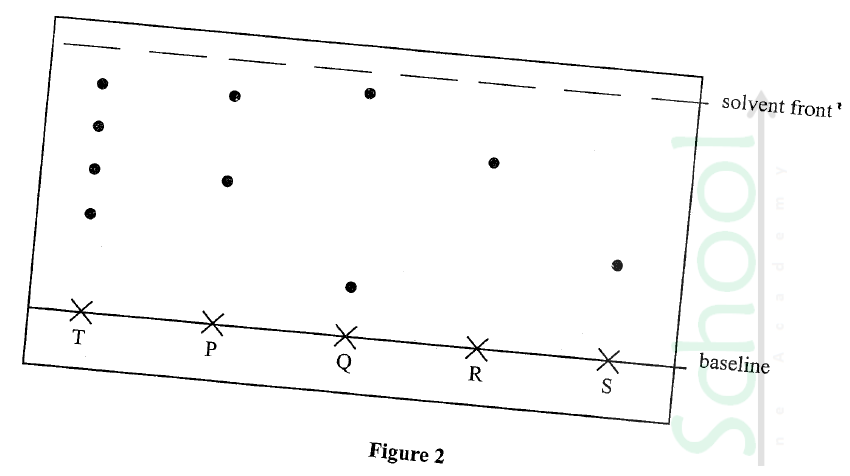
An experiment was Carried out to determine the presence of Substances P, Q, R and S in mixture T. The results obtained are shown in Figure 2.
(a) Ne the method of separation illustrated in Figure 2.
(b) Select: (i) one Substance Which Contains a Component not present in T. (ii) a substance which is least Soluble in the solvent used.
ANSWERS
(a)Chromatography/paper chromatography
(b)(i)Q (ii)S
Explain how a student can establish whether a liquid sample extracted from a plant is pure.
ANSWERS
When a student was stung by a nettle plant, a teacher applied an aqueous solution of ammonia to the affected area of the skin and the
student was relieved of pain. Explain.
ANSWERS
Describe an experimental procedure that can be used to extract oil from nut seeds.
ANSWERS
Crush grind using a pestle and mortar, add suitable solvent of propanone ethanol alcohol and stir to dissolve oil. Filter the mixture to obtain a solution of the oil. Leave the solution in the sun for propanone to evaporate leaving the oil.
Name an appropriate apparatus that is used to prepare standard solutions in the laboratory. (1 mark)
Expected Response
volumetric flask
Related Chemistry Questions and Answers on Simple Classification of Substances Form 1 Level
Iron (III) oxide was found to be contaminated with copper (II) sulphate. Describe how a pure sample of iron (III) oxide can be obtained.
ANSWERS
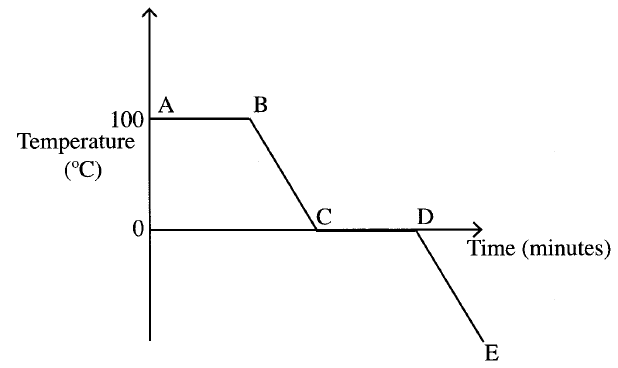
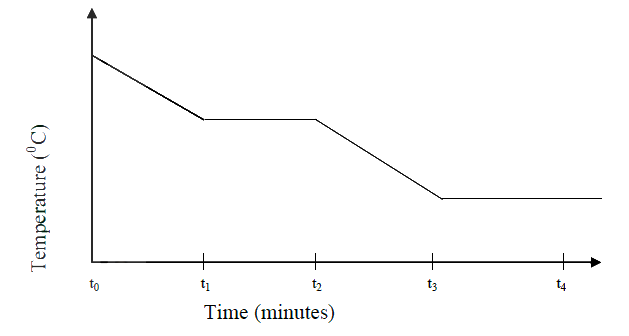
The graph below is a cooling curve for water. Study it and answer the questions that follow
(a) Explain what happens to the molecules of water in the region BC in terms of kinetic theory.
(b) In what state is the water in the region DE?
ANSWERS
The molecules of water are
(a) Loosing heat . The kinetic energy decreases and the molecules move closer to each other (b) Solid state
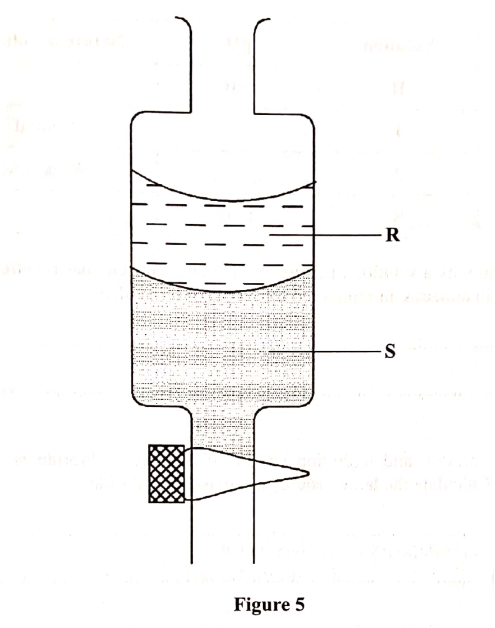
Figure 5 shows an apparatus used to separate a mixture of water and hexene.
The set up below was used to separate a mixture of methanol and propanol. Study it and answer the question that follow.
a) State the function of X
b) Which liquid will collect first in the beaker? give a reason.
ANSWERS
Draw a set up that can be used to separate a mixture of sand and iodine
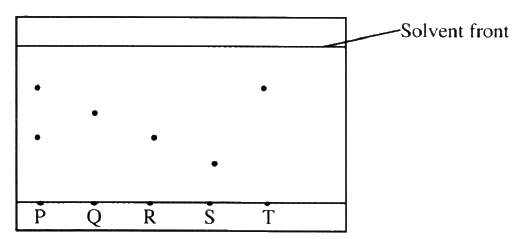
The chromatogram below was obtained from a contaminated food sample P.
Contaminants Q, R, S and T are suspected to be in P. Use it to answer the following questions.
a) Identify the contaminants in mixture P.
b) Which is the most soluble contaminant in P.?
Describe an experiment procedure that can be used to extract oil from nut seeds
ANSWERS
Give a reason why phosphorus is stored under water.
Expected Response
To keep away air/ oxygen which would react with it
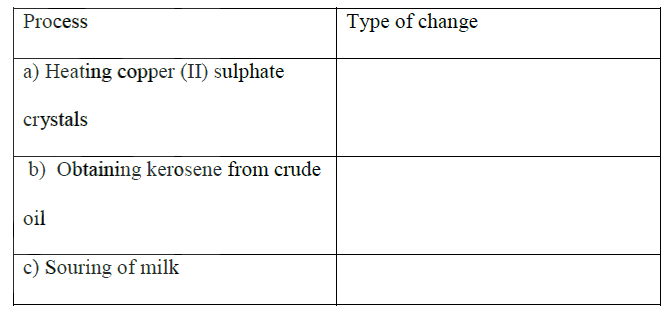
Explain the change in mass that occurs when the following substances are separately heated in open crucibles
(a) Copper metal (b) Copper (II) nitrate
Expected Response
a) Mass increases because oxygen combine with copper metal
b) Mass decreases it decomposes into gases that escape.
A sample of water in a beaker was found to boil at 101.5°C at 1 atmospheric pressure.
Assuming that the thermometer was not faulty, explain this observation.
ANSWERS
Iron (III) oxide was found to be contaminated with copper (II) sulphate. Describe how a pure sample of iron (III) oxide can be obtained.
A mixture contains ammonium chloride, copper (II) oxide and sodium chloride.
Describe how each o the substances can be obtained from the mixture.
ANSWERS
Heat the mixture to sublime the ammonium chloride.
Add water to dissolve the sodium chloride ; copper (ii) oxide does not dissolve Filter and evaporate the filtrate to obtain sodium chloride.
Hydrate cobalt(II) chloride exists as pink crystals and anhydrous cobalt(II) chloride is a blue powder. Describe a laboratory experiment that can be used to show that the action of heat on hydrated cobalt(II) chloride is a reversible reaction.
ANSWERS
(a) Chemical change
(b) Physical change (c) Chemical change
The diagram below shows the physical state of matter. Study it and answer the questions that follow
Identify the processes R, V, w and U
(c) Name one substance which can undergo the process represented by S and T.
Expected Response
Expected Response
Expected Response
ANSWERS
(a)Cooling
(b)Latent heat of fusion |
Chemistry Topics
All
Archives
December 2024
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
|
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |




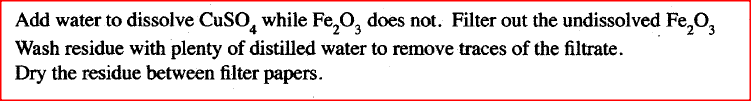


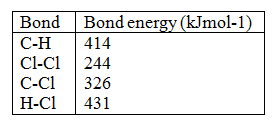


 RSS Feed
RSS Feed

