KCSE CHEMISTRY QUESTIONS AND ANSWERS PER TOPIC
|
The table below shows the volumes of nitrogen dioxide gas produced when different volume of 1M nitric acid were each reacted with 2.07 g of lead at room temperature.
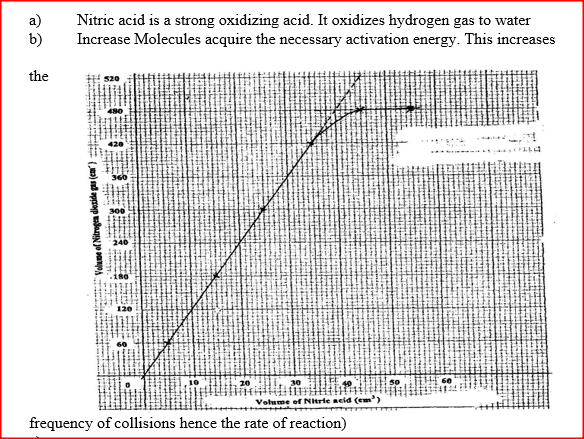
a) Give a reason why nitric acid is not used to prepare hydrogen gas. b) Explain how the rate of the reaction between lead and nitric acid would be affected if the temperature of the reaction mixture was raised. c) On the grid provided below, plot a graph of the volume of the gas produced (Vertical axis) against volume of acid.
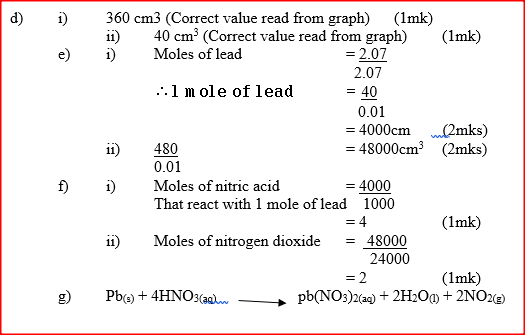
d) Using the graph, determine the volume of
:i) Nitrogen dioxide produced when 30cm3 of 1 M nitric acid were reacted with 2.07 g of lead ii) 1M nitric acid which would react completely with 2.07g of lead. e) Using the answer in d(i) above, determine: i) The volume of 1M nitric acid that would react completely with one mole of lead (Pb=207) ii) The volume of nitrogen dioxide gas produced when one mole of lead reacts with excess 1 M nitric room temperature. f) Calculate the number of moles of: i) 1M nitric acid that reacted with one mole of lead ii) nitrogen dioxide produced when one mole of lead were reacted with excess nitric acid. (Molar gas volume of 2400cm3) g) Using the answers obtained in f (i) and (ii) above, write the equation for the reaction between lead and nitric acid given that one mole of lead nitrate and two moles of water were also produced. Related Chemistry Questions and Answers on Reaction Rates and Reversible Reactions Form 4 Level
0 Comments
Leave a Reply. |
Chemistry Topics
All
Archives
December 2024
|
We Would Love to Have You Visit Soon! |
Hours24 HR Service
|
Telephone0728 450425
|
|
8-4-4 materialsLevels
Subjects
|
cbc materialsE.C.D.E
Lower Primary
Upper Primary
Lower Secondary
Upper Secondary
|
teacher support
Other Blogs
|
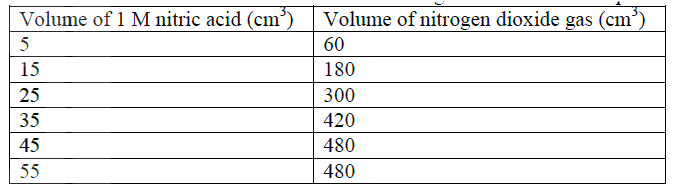

 RSS Feed
RSS Feed