|
(a) Methanol is manufactured from carbon (IV) oxide and hydrogen gas according to the equation:
The reaction is carried out in the presence of a chromium catalyst at 700K and 30kPa. Under these conditions, equilibrium is reacted when 2% of the carbon (IV) oxide is converted to methanol
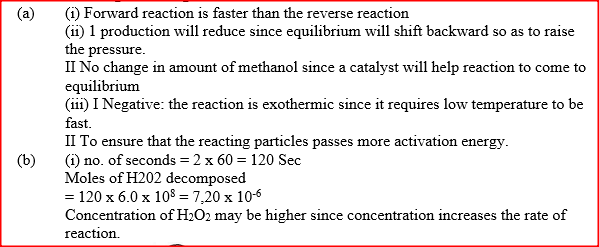
(i) How does the rate of the forward reaction compare with that of the reverse reaction when 2% of the carbon (IV) oxide is converted to methanol? (ii) Explain how each of the following would affect the yield of methanol: I Reduction II Using a more efficient catalyst (iii) If the reaction is carried out at 500K and 30kPa, the percentage of carbon (IV) oxide converted to methanol is higher than 2% I what is the sign of ΔH for the reaction? Give a reason II Explain why in practice the reaction is carried out at 700K but NOT at 500K (b) Hydrogen peroxide decomposes according to the following equation : 2H2O2(aq) →2H2O(l) + O2 (g) In an experiment, the rate of decomposition of hydrogen peroxide was found to be 6.0 x 10-8 mol dm-3 S-1. (i) Calculate the number of moles per dm3 of hydrogen peroxide that had decomposed within the first 2 minutes (ii) In another experiment, the rate of decomposition was found to be 1.8 x 10-7 mol dm-3S-1. The difference in two rates could have been caused by addition of a catalyst. State, giving reasons, one other factor that may have caused the difference in two rates of decomposition Related Chemistry Questions and Answers on Reaction Rates and Reversible Reactions Form 4 Level
0 Comments
Leave a Reply. |
Chemistry Topics
All
Archives
December 2024
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
|
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |
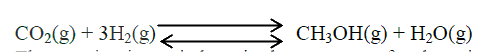

 RSS Feed
RSS Feed

