A crystal of potassium permanganate was carefully introduced at the bottom of water column held in a gas jar. After sometime, the whole volume of water was coloured. Explain this observation (2mks)
The continuously moving water molecules hit the crystal from all directions causing it to split into the tiny particles of which it consist. The same movement caused the particles to diffuse to all parts of the liquid, this rendering the whole volume colored.
0 Comments
K.C.S.E Physics Q & A - MODEL 2019PP1QN17
(a) State what ¡s meant by Brownian Motion.
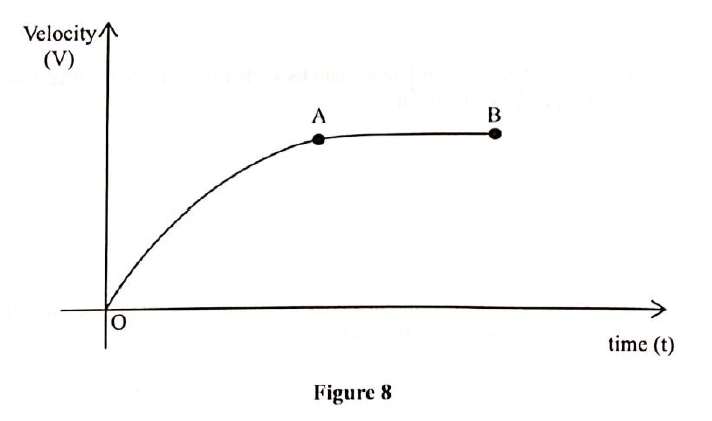
(b) Figure 8 shows the graph of velocity against time For a small steel ball Falling in a viscous liquid.
(i) Describe the motion of the steel ball as represented by part OA
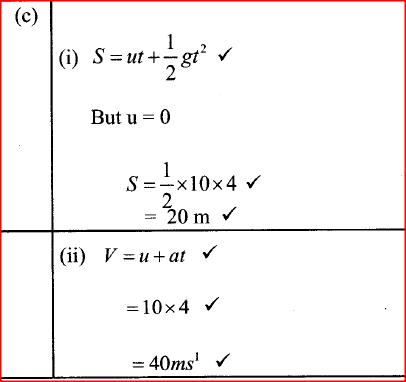
(ii) Explain why the velocity of A and B is constant (c)A student throws a tennis ball vertically upwards from the ground and it lands back after 8 seconds.( acceleration due to gravity g =10m/s2) Determine the: (i) maximum height reached by the ball iii) velocity with which the ball hits the ground.
K.C.S.E Physics Q & A - MODEL 2018PP1QN05
It is observed that a drop of milk carefully put into a cup of water turns the water white after some time. State the reason for this observation.
answer
K.C.S.E Physics Q & A - MODEL 2015PP1QN06
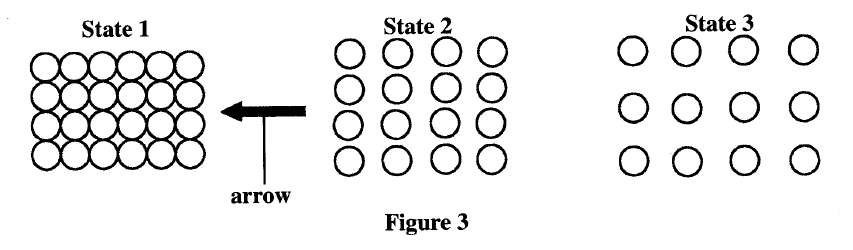
Figure 3 shows the arrangement of molecules in the three states of matter.
(a) Name the process represented by the arrow.
(b) State the reason for the arrangement of molecules in state 3.
ANSWER
(a) - freezing
(b) - The intermolecular forces are weaker K.C.S.E Physics Q & A - MODEL 2014PP1QN14
In a smoke cell experiment to demonstrate Brownian motion, smoke particles are seen moving randomly. State the cause of the randomness.
answer
K.C.S.E Physics Q & A - MODEL 2013PP1QN13
A drop of blue ink is introduced at the bottom of a beaker containing water. It is observed that after some time, all the water in the beaker turns blue. Name the process that takes place.
answer
K.C.S.E Physics Q & A - MODEL 2012PP1QN04
A bottle containing a smelling gas is opened at the front bench of a classroom.
State the reason why the gas is detected throughout the room.
ANSWER
K.C.S.E Physics Q & A - MODEL 2011PP1QN10
State the reason why it is easier to separate water into drops than to separate a solid into smaller pieces.
ANSWER
K.C.S.E Physics Q & A - MODEL 2009PP1QN06
Two identical beakers A and B containing equal volumes of water are placed on a bench. The Water in A is cold while in B it is warm. Identical pieces of potassium permanganate are placed gently at the bottom of each beaker inside the water. It is observed that the spread of colour in B is faster than in A. Explain this observation.
answer
K.C.S.E Physics Q & A - MODEL 2008PP1QN09
Explaining the difference between a liquid and a gas in terms of intermolecular distances and forces.
answer
K.C.S.E Physics Q & A - MODEL 2007PP1QN15
Brown motion of smoke particles can be studied by using the apparatus shown in figure 9 to observe the motion, some smoke is enclosed in the smoke cell and then observed through the microscope.
(a) Explain the role of the smoke particle, lens and microscope in the experiment
Smoke particles Lens (b) State and explain the nature of the observed motion of the smoke particles (c) State what will be observed about the motion of the smoke particles if the temperature surrounding the smoke cell is raised slightly.
K.C.S.E Physics Q & A - MODEL 2003PP1QN05
Explain the cause of random motion of smoke particles as observed in Brownian motion experiment using a smoke cell.
answer
K.C.S.E Physics Q & A - MODEL 2002PP1QN06
When an inflated balloon is placed at equal in a refrigerator it is noted that its volume reduces. Use the kinetic theory of gases to explain this observation.
answers
K.C.S.E Physics Q & A - MODEL 1999PP1QN07
In an experiment to study the atoms of gold, a beam of alpha- particles was directed onto a thin sheet of gold. The following observations were made:
(i) Majority of the particles went straight through undeflected (ii) A few particles deflected through varying angles up to 180.
K.C.S.E Physics Q & A - MODEL 1996PP1QN37
In the Brownian motion experiment, smoke particles are observed to move randomly. Explain how this motion is caused
ANSWERS
|
CATEGORIES
Categories
All
Topics
FORM I - PHYSICS SYLLABUSFORM II - PHYSICS SYLLABUSTOPICS
FORM III - PHYSICS SYLLABUSFORM IV - PHYSICS SYLLABUSARCHIVES
RSS FEEDS
AUTHOR
M.A NyamotiMy passion is to see students pass using right methods and locally available resources. My emphasis is STEM courses
|
We Would Love to Have You Visit Soon! |
Hours24 HR Service
|
Telephone0728 450425
|
|
8-4-4 materialsLevels
Subjects
|
cbc materialsE.C.D.E
Lower Primary
Upper Primary
Lower Secondary
Upper Secondary
|
teacher support
Other Blogs
|



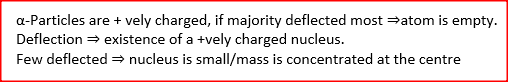
 RSS Feed
RSS Feed