K.C.S.E Physics Q & A - MODEL 2019PP1QN18
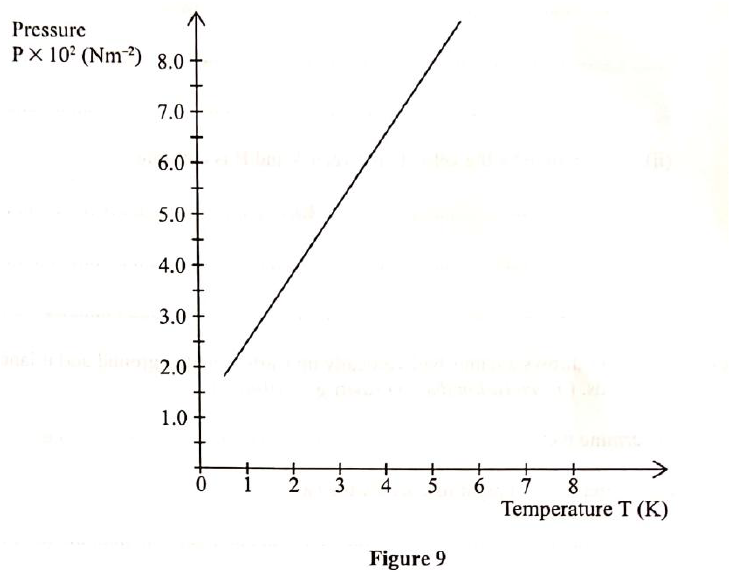
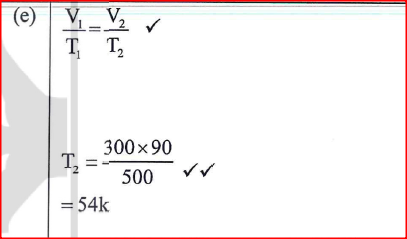
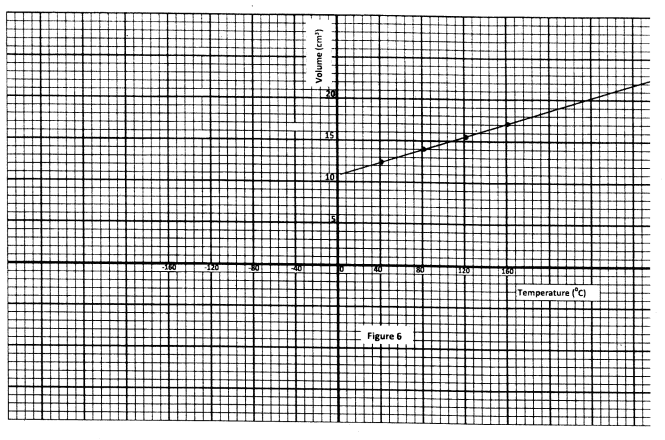
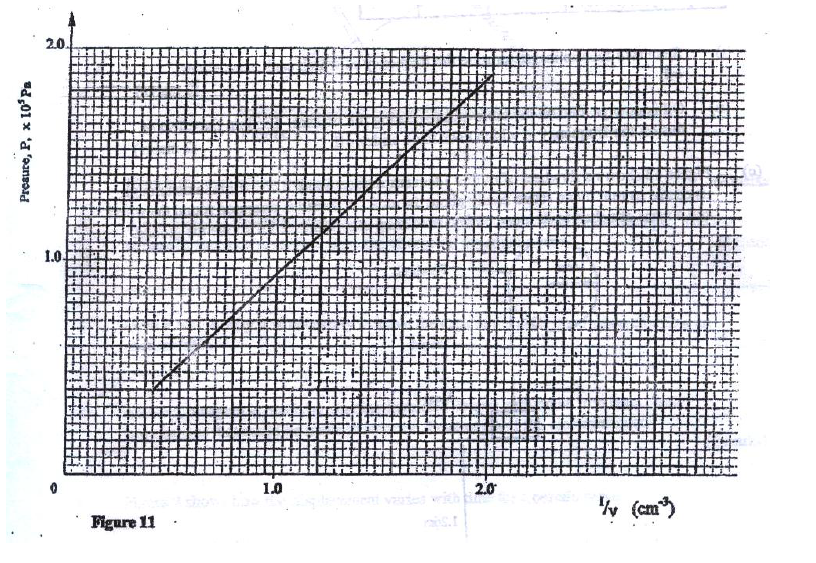
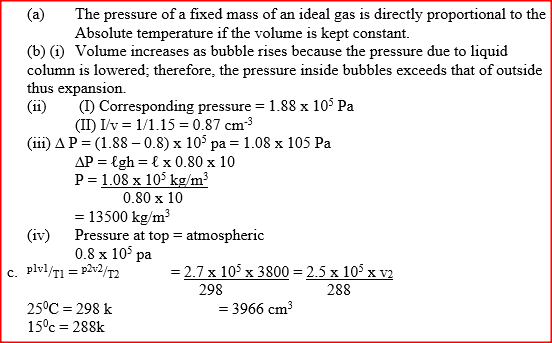
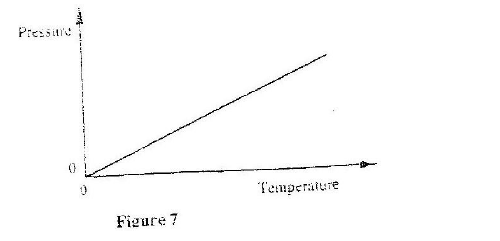
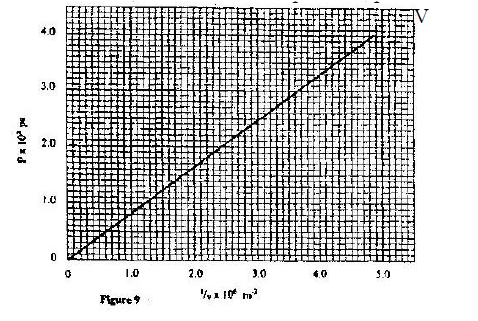
(a) Figure 9 shows a graph of pressure against temperature for a fixed mass of gas at constant volume.
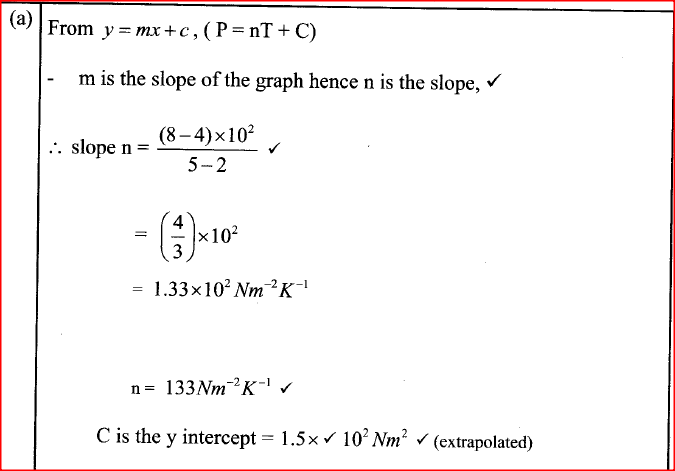
From the graph, determine the values of n arid e given that P = nT + c where n and c are constants.
|
CATEGORIES
Categories
All
Topics
FORM I - PHYSICS SYLLABUSFORM II - PHYSICS SYLLABUSTOPICS
FORM III - PHYSICS SYLLABUSFORM IV - PHYSICS SYLLABUSARCHIVES
RSS FEEDS
AUTHOR
M.A NyamotiMy passion is to see students pass using right methods and locally available resources. My emphasis is STEM courses
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
|
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |

























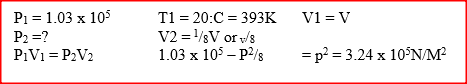
 RSS Feed
RSS Feed

