K.C.S.E Physics Q & A - MODEL 2018PP1QN18
(a) State two quantities that must be kept constant in order to verify Boyle’s law.
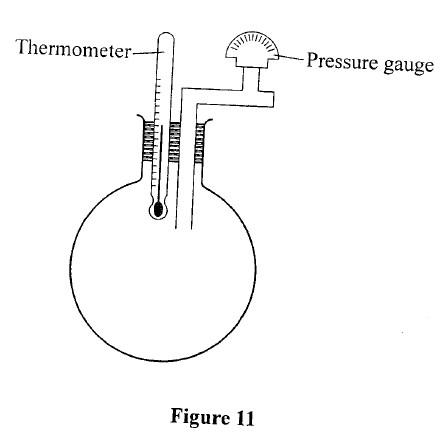
(b) An air bubble at the bottom of a beaker full of water becomes larger as it rises to the surface. State the reason why; (i) the bubble rises to the surface, (ii) It becomes larger as it rises. (c) State two assumptions made in explaining the gas laws using the kinetic theory of gases. (d) Figure 11 shows an incomplete experimental set up that was prepared by a student to verify one of the gas laws. (i) State with a reason which one of the laws may be verified using the set up.
|
CATEGORIES
Categories
All
Topics
FORM I - PHYSICS SYLLABUSFORM II - PHYSICS SYLLABUSTOPICS
FORM III - PHYSICS SYLLABUSFORM IV - PHYSICS SYLLABUSARCHIVES
RSS FEEDS
AUTHOR
M.A NyamotiMy passion is to see students pass using right methods and locally available resources. My emphasis is STEM courses
|
We Would Love to Have You Visit Soon! |
Hours24 HR Service
|
Telephone0728 450425
|
|
8-4-4 materialsLevels
Subjects
|
cbc materialsE.C.D.E
Lower Primary
Upper Primary
Lower Secondary
Upper Secondary
|
teacher support
Other Blogs
|
 RSS Feed
RSS Feed