K.C.S.E Physics Q & A - MODEL 2019PP1QN16
(a) State the meaning of the term “heat capacity.”
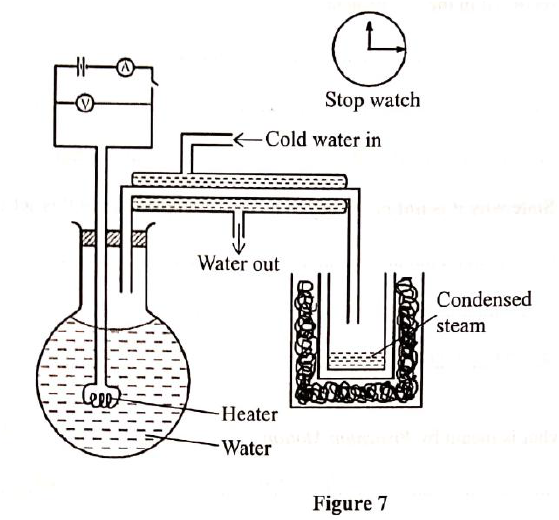
(b) State how pressure affects the melting point of a substance. (c)Figure 7 shows a set tip of apparatus that may be used to apparatus the specific latent heat of vaporization of steam
(I) Describe how the mass of condensed steam is determined.
(ii) Other than mass and time, state two other measurements that should be taken during the experiment. (iii) Show how the measurements in (c)(ii) can be used to determine the specific latent heat of vaporization of water. (iv) State the precaution that should be taken so that the mass of the condensed steam measured corresponds to the actual mass of steam collected during the time recorded in the experiment (v) State why it is not necessary to measure temperature in in the set up
answers
(a) Heat capacity is the quantity of heat energy required to raise the temperature of a substance by 1K.
(b) - Increase in pressure lowers the melting point while decrease in (1 mark) pressure raises/increases the melting point. (c) (i) - Measure the mass of the empty beaker M1. Measure the mass of the beaker plus the condensed steam M2 Get the difference between the two masses (M2 —M1) = M (ii) - Voltage Current
(iv) - Start timing when the steam drops start forming out steadily and Stop immediately the beaker is withdrawn.
(V) Steam is produced at boiling point where temperature is constant.
0 Comments
Leave a Reply. |
CATEGORIES
Categories
All
Topics
FORM I - PHYSICS SYLLABUSFORM II - PHYSICS SYLLABUSTOPICS
FORM III - PHYSICS SYLLABUSFORM IV - PHYSICS SYLLABUSARCHIVES
RSS FEEDS
AUTHOR
M.A NyamotiMy passion is to see students pass using right methods and locally available resources. My emphasis is STEM courses
|
We Would Love to Have You Visit Soon! |
Hours24 HR Service
|
Telephone0728 450425
|
|
8-4-4 materialsLevels
Subjects
|
cbc materialsE.C.D.E
Lower Primary
Upper Primary
Lower Secondary
Upper Secondary
|
teacher support
Other Blogs
|

 RSS Feed
RSS Feed