|
These are chemistry questions and answers categorized according to topics, papers i.e. Paper 1 and 2, Levels i.e. form 1 to form 4, kcse year the examination was done and section A or B
Select topic/category to open topical questions from that particular option provided. Chemistry Topics
0 Comments
The empirical formula of a hydrocarbon is (CH2). It has a density of 0.001167g/cm3 at room temperature and pressure. (Molar gas volume at r.t.p is 24dm3)a) Determine the molecular formula of the hydrocarbon (3mks)
b) Draw the structural formula of the hydrocarbon (1mk)c) Ethene gas burns in Oxygen to form Carbon (IV) oxide and water.i) Write an equation for the reaction between ethane gas oxygen gas (1mk)ii) 15cm3 of ethene gas were mixed with 50cm3 of oxygen gas and the mixture was ignited into complete combustion. Calculate the volume of excess unreacted gas (3mks)d) What happens when ethene gas is bubbled through bromine water? (2mks)
e) Give any two uses of ethene gas (2mks)
Uses of Ethene gas
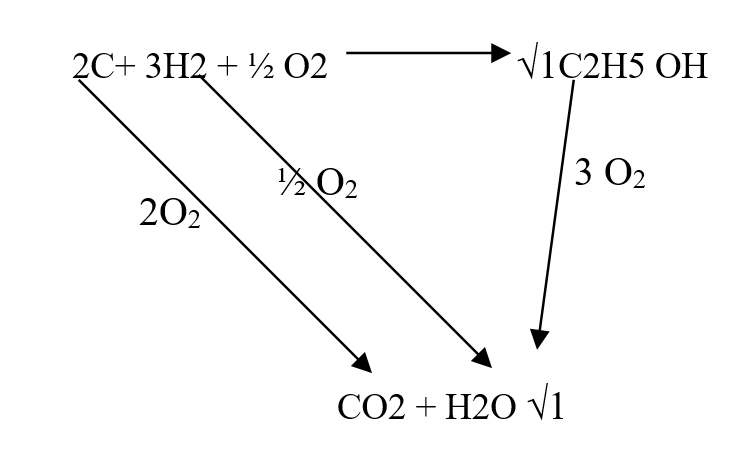
Use the information below to answer the questions that follow Ethanol is formed as shown below11/10/2021 Use the information below to answer the questions that follow Ethanol is formed as shown belowDraw the energy cycle diagram and for the formation and combustion of ethanol and calculate the heat of formation of ethanol (3mks)a) Explain why sulphur is soluble in ethanol but hot in water (1mk)
b) Explain how a pure sample of sodium chloride can be obtained from a mixture of the two (1mk)
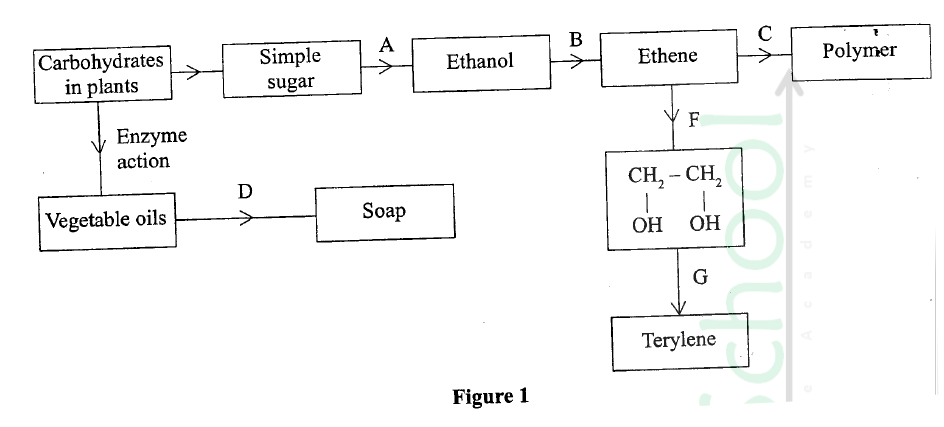
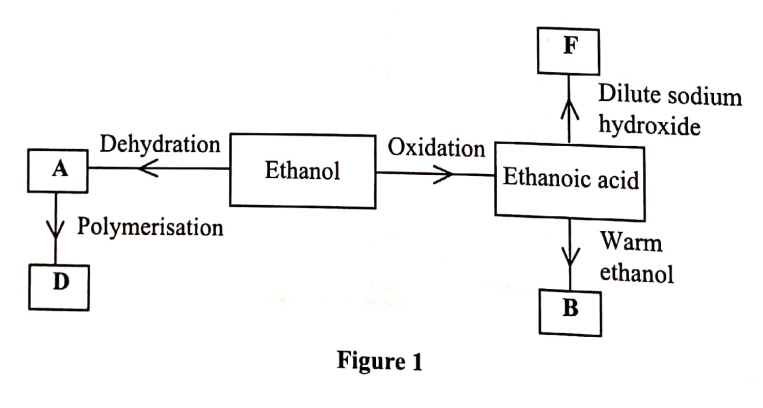
The diagram in figure I shows some natural and industrial processes. Study it and answer the questions that follows
(a) Identify the processes labelled:
A................... B................... C................... D................... (b) State the reagents and conditions required for processes B and D. (i) Process B: Reagent................ Conditions ............ (ii) Process D: Reagent................ Conditions ............ (iii) Describe how process D is carried out. (iv) State two additives used to improve the quality of soap. (c) State the reagents required in steps F and G. (iii) Draw the structure of terylene. (d) (i) Name the polymer formed in step C. (ii) State one disadvantage of the polymer formed in (d) (i).
When ethene gas is compressed at a high temperature, a solid is formed.
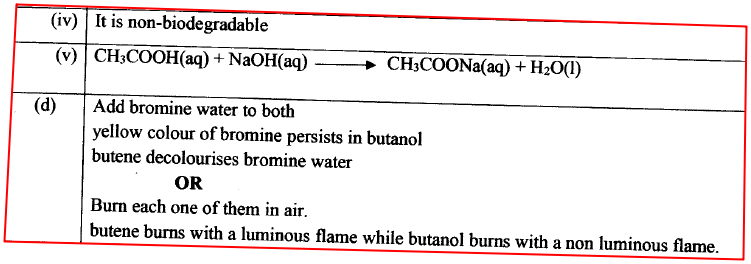
(a) Give the name of the solid. (b) Explain why it is not advisable to allow the solid to accumulate in the environment.
ANSWERS
(a)Polythene / Polyethene
(b)It is non-biodegradable, hence pollutes the environment.
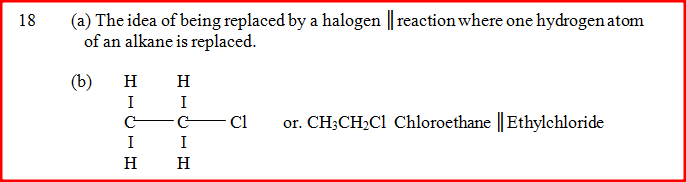
The following are formulae of organic compounds. Use the formulae to answer the questions that follow:
(a) Select:
(i) two compounds which when reacted together produce a sweet smelling compound. (ii) an unsaturated hydrocarbon. (b) Name the compound selected in (a) (ii).
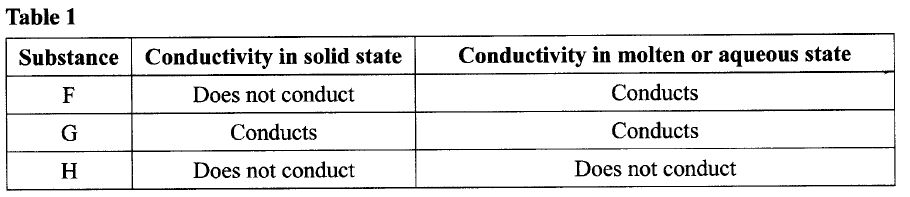
The conductivity of some substances was investigated. The observations made were recorded in
Table 1. Use it to answer the questions that follow.
(a) (i) Identify a substance that is a metal. Give a reason.
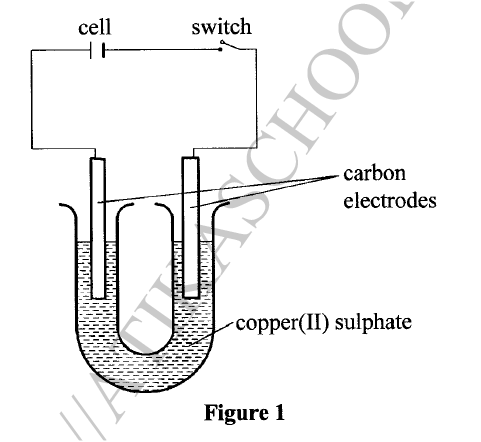
(ii) Substance F does not conduct electricity in solid state but conducts in molten or aqueous state. Explain. (b) Copper(II) sulphate solution was electrolysed using the set up in Figure 1.
(i) State the observations made during electrolysis.
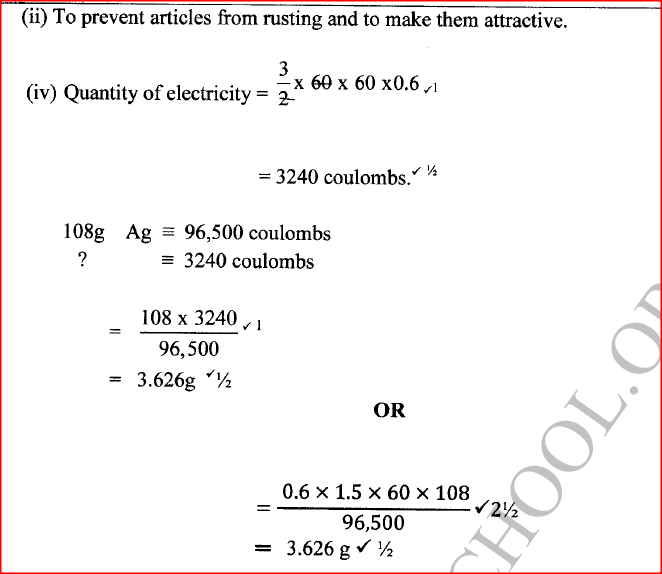
(ii) Write the equation for the reaction that occurs at the anode. (iii) State the expected change in pH of the electrolyte after electrolysis. (c) The experiment was repeated using copper electrodes instead of carbon electrodes. Describe the observations made at each electrode. (d) Electroplating is an important industrial process. What is meant by electroplating. (iii) During electroplating of an iron spoon, a current of 0.6 amperes was passed through aqueous silver nitrate solution for 1 1/2 hours. Calculate the mass of silver that was deposited on the spoon. (Ag = 108.0 ; I F = 96,500 C /mol)
ANSWERS
(a)(i) G — Contains delocalized electrons present in solid and molten state.
(ii) In solid state, the ions are rigidly held in position and cannot move, hence will not conduct. In molten/aqueous state, the ions are mobile and will be able to conduct electric current. (b)(i)The blue electrolyte fades and finally changes from blue to colourless, Effervescence / bubbles of a colourless gas. A brown deposit forms on the cathode.
(c) With copper electrodes:
Anode will go into solution as copper ions hence it decreases in mass/size. Brown deposit forms at the cathode hence the cathode increases in mass. (d) (i) This is the coating of an article / object with another metal by electrolytic method /electrolysis.
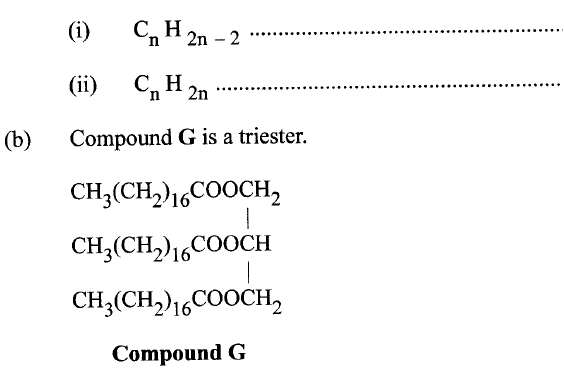
(a) Name the homologous series represented by each of the following general formulae.
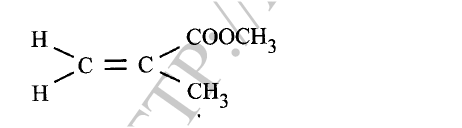
(i) Give the physical state of compound G at room temperature.
(ii) G is completely hydrolysed by heating with aqueous sodium hydroxide. I Give the structural formula of the alcohol formed. II Write a formula for the sodium salt formed. III State the use of the sodium salt. (c) Ethyne is the first member of the alkyne family. (i) Name two reagents that can be used in the laboratory to prepare the gas. (ii) Write an equation for the reaction. (d) Perspex is an addition synthetic polymer formed from the monomer,
(i) What is meant by addition polymerisation?
(ii) Draw three repeat units of perspex. Give one use of perspex (iv) State two environmental hazards associated with synthetic polymers.
ANSWERS
When an aqueous solution of compound X was mixed with a few drops of bromine water, the colour of the mixture remained yellow.
When another portion of solution X was reacted with acidified potassium dichromate(VI), the colour of the mixture changed from orange to green. (a) What conclusion can be made from the use of: (i) bromine water? (ii) acidified potassium dichromate(VI)? (b) Solution X was reacted with a piece of a metal and a colourless gas was produced. Describe a simple experiment to identify the gas.
Study the flow chart in Figure 5 and answer the questions that follow.
(a) Identify substances K and L. K:
(b) Name one reagent that can be used to carry out process J.
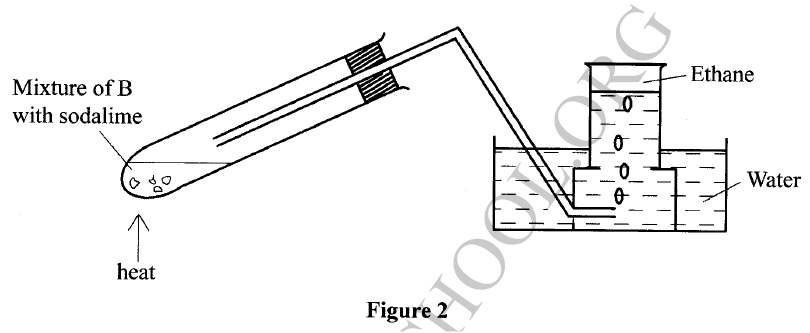
The set-up in Figure 2 was used to prepare a sample of ethane gas. Study it and answer the questions that follow.
(a) Name B
(b) Write an equation for the complete combustion of ethane. (c) State one use of ethane.
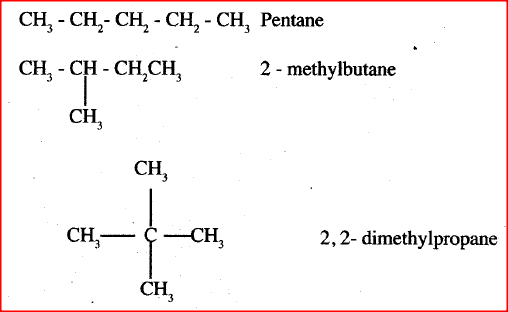
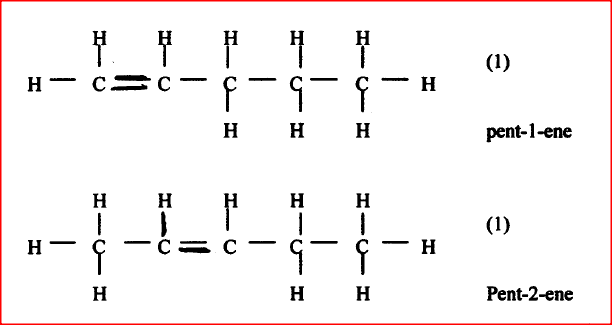
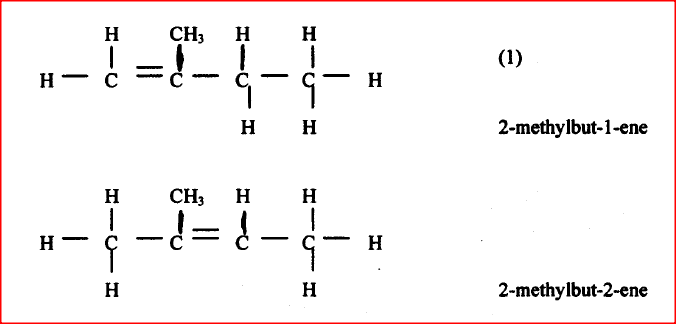
Draw and name the isomers of pentane.
1.
(a) Alkanes are said to be saturated hydrocarbons.
(b) When the alkane, hexane, is heated to high temperature, one of the products is ethene.
Related Chemistry Questions and Answers on Organic Chemistry II Form 3 Level
(a) State one source of alkanes.
(b) Ethane gas was reacted with 1 mole of bromine gas. State one observation made during this reaction.
ANSWERS
(a) Sources of alkanescrude oil/petroleum natural gas/biogas
(b) The brown/red/orange/yellow colour of bromine is discharged/discoloured
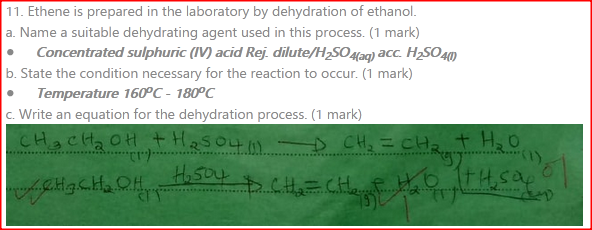
Ethene is prepared in the laboratory by dehydration of ethanol.
A monomer has the following structure.
(a) Draw the structure of its polymer that contains three monomers. (1 mark)
(b) A sample of the polymer formed from the monomer has a molecular mass of 4992. Determine the number of monomers that formed the polymer (C= 12; H= 1.0). (2 marks)
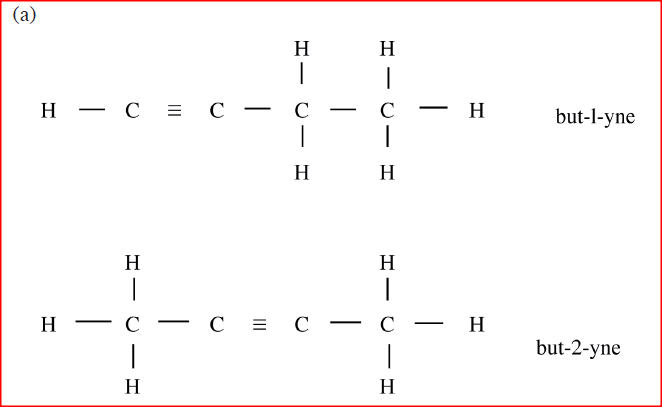
(a) Draw and name the isomers of butyne
(b) State one use of polystyrene.
In the presence of U.V light, ethane gas undergoes substitution reaction with chlorine.
(a) What is meant by the term? Substitution reaction: (b) Give the structural formula and the name of the organic product formed when equal volumes of ethane and chlorine react together.
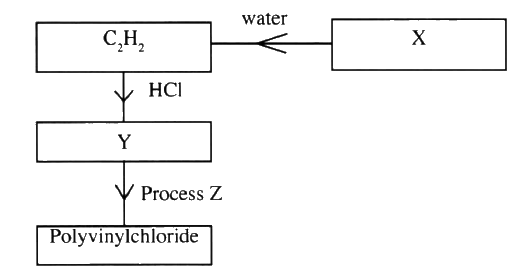
Study the flowchart below and answer the questions that follow:
(a) Identify:
(i) X (ii) Y (b) State two uses of polyvinylchloride
Draw and name the isomers of pentane.
A hydrocarbon P was found to decolourise bromine water. On complete combustion of 2 moles of P, 6 moles of carbon dioxide and 6 moles of water were formed
Exhaust fumes of some cars contain carbon(II)oxide and other gases.
(a) Explain how carbon (II) oxide is formed in the internal combustion engines. (b) Name two gases other than carbon (II) oxide that are contained in exhaust fumes and are pollutants.
ANSWERS
(a) Carbon (II) oxide is formed in the internal combustion engines when fuel burns under limited oxygen.
(b) Pollutant gas - Carbon (IV) oxide, Nitrogen (1V) oxide and Sulphur (IV) oxide.
Draw the structures and give the name of the three alkaline having molecular formula C5H10
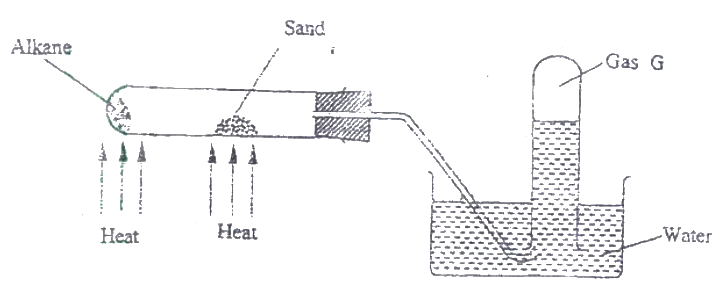
The figure below represents the set up that was used to crack an alkane.
a) What was the purpose of the sand?
b) After some time, a colourless gas G collected in the test-tube. Describe a chemical test and the observations that would be made in ordered to identify the class of compounds to which gas G belongs.
ANSWERS
(a) Catalyst or words to that effect
(b) Add bromine water or acidified potassium magnate (VII) if they decolorize then gas is either an alkene or an alkynes |
Chemistry Topics
All
Archives
December 2024
|
Can't find what you are looking for? Don't worry, Use the Search Box Below.
|
Primary Resources
College Resources
|
Secondary Resources
|
Contact Us
Manyam Franchise
P.O Box 1189 - 40200 Kisii Tel: 0728 450 424 Tel: 0738 619 279 E-mail - sales@manyamfranchise.com |
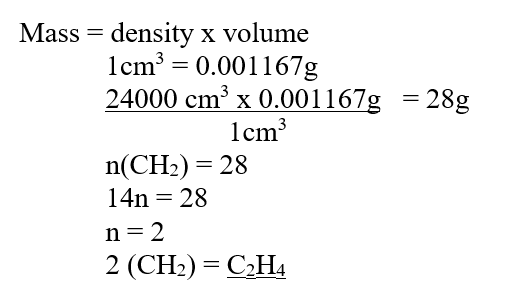
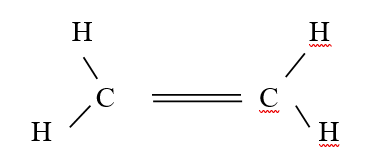
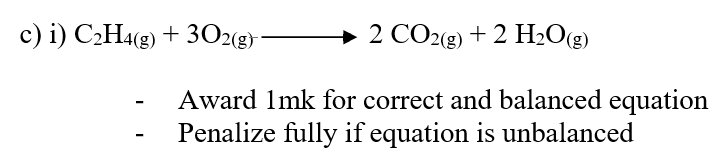
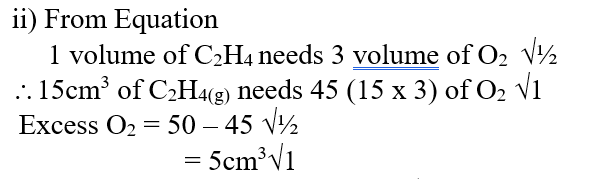
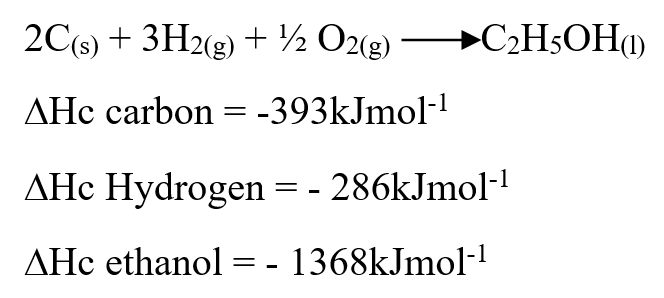
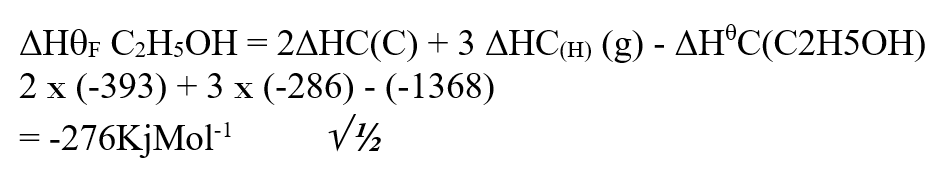
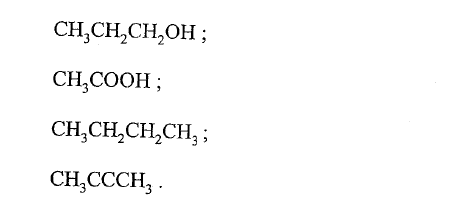

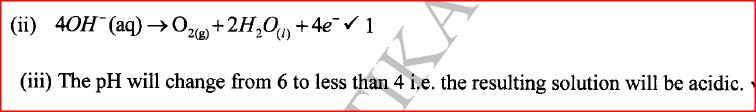









 RSS Feed
RSS Feed

